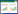目前,世界肥料正在向高浓度、液体化、缓效化、高利用率方向发展,多组分水溶性肥料是一种养分含量高且营养全面、可自动化施肥的高效肥料,是未来肥料发展的主要方向之一[1-3]。共结晶技术因能改善晶体产品的形状、流动性、吸湿性和稳定性成为生产含多种元素的水溶性肥料的重要手段[4-6]。结晶过程的设计需要有精确溶解度数据,因此,要用共结晶法生产水溶肥,其固-液相平衡数据测定必不可少[7, 8]。利用相平衡数据绘制的相图可作为理论工具指导实践,为结晶过程提供必要的热力学数据。
不同温度下多组分体系的固-液相平衡对多组分共结晶研究提供了重要的热力学数据[9, 10]。NH4H2PO4、KH2PO4、KCl、NH4Cl、(NH2)2CO是生产水溶性肥料的主要原料[11, 12]。本课题组已对NH4H2PO4、KH2PO4、KCl、NH4Cl、(NH2)2CO的多元体系的多个温度的固液相平衡进行了研究,如胡雪等[13, 14]研究了三元体系KCl-(NH2)2CO-H2O、NH4H2PO4-(NH2)2CO-H2O和交互四元系K+, NH4+//Cl-, H2PO4--H2O及其三元子体系在283.15 K下的相平衡;杨家敏[15, 16]、吴强[17]、邓文清[18]、樊小娟[19]、王肖丽[20]、黄林川[21, 22]、陈艳[23]等研究了交互五元体系K+, NH4+//Cl-, H2PO4-, (NH2)2CO-H2O及其子体系在283.15、298.15、313.15和353.15 K下的相平衡;其他研究者也对这些组分不同温度下的相平衡进行了研究,如张逢星等[24]研究了四元体系K+//Cl-, H2PO4-, (NH2)2CO-H2O在298.2 K下的相平衡;Yu[25]、赵长伟[26]和Zhao[27]等研究了三元体系KCl-NH4Cl-H2O在298.15、308.15和353.15 K下的相平衡;Shi等[28]研究了三元体系KCl-KH2PO4-H2O在298.15 K下的相平衡;Zhang[29]等研究了三元体系KH2PO4-NH4H2PO4-H2O在283.15和313.15 K下的相平衡。
对于三元体系NH4H2PO4-NH4Cl-H2O,已有人研究了258.15[30]、273.15[30]、283.15[17]、293.15[30]、298.15[30, 31]、308.15[30]、313.15[32]和353.15 K[22]下的固液相平衡关系。而对于该体系的共结晶过程研究需要更广泛温度下的溶解度数据,因此本研究采用等温溶解平衡法研究三元体系NH4H2PO4-NH4Cl-H2O在303.15、323.15、333.15和343.15 K下的固液相平衡关系,并在获得的溶解度数据基础上绘制相应的相图,同时采用Pitzer-Harvie-Weare模型对该体系进行了溶解度数据的关联计算。
1 实验部分 1.1 试剂及仪器实验试剂:NH4H2PO4(AR,质量分数≥99%,成都金山化学试剂有限公司);NH4Cl(AR,质量分数≥99%,天津永大化学试剂有限公司);对二甲氨基苯甲醛、钼酸铵(AR,质量分数≥99%,天津科密欧化学试剂有限公司);抗坏血酸(AR,质量分数≥99%,国药集团化学试剂有限公司)。实验过程中所用的水均为自制去离子水,电阻率为18.25 MΩ·cm。
实验仪器:电子天平(BSM型,上海卓精电子科技有限公司,精度0.000 1 g);低温恒温槽(DC-4006型,上海菁海仪器有限公司,控温精度±0.1 K);恒温磁力搅拌器(S10-3型,上海司乐仪器有限公司);紫外可见分光光度计(TU-1810型,北京普析通用仪器有限责任公司);X射线衍射仪(XRD)(X’Pert3 Powder型,荷兰帕纳科);电热鼓风干燥箱(GZX-9146MBE,上海博讯实业有限公司医疗设备厂);氦氖激光器(GY-11型,天津拓普仪器有限公司);激光器功率指示仪(WGN-1型,天津拓普仪器有限公司); 同步热分析仪(NETZSCH-STA 499 F3,德国-耐驰)。
1.2 实验方法实验采用等温溶解平衡法[33]。实验装置如图 1所示,在整个实验过程中,系统温度由低温恒温槽保持在设定温度±0.1 K下。以303.15 K为例,在500 mL的夹套玻璃结晶器中加入去离子水50 g、过量的NH4Cl(NH4H2PO4)和一定量的NH4H2PO4(NH4Cl);用橡胶塞密封并置于恒温磁力搅拌器上,结晶器连接低温恒温槽设定温度在303.15(±0.1) K并开启搅拌。在溶液达到平衡后,停止搅拌并静置10 min,利用在电热鼓风干燥箱中已预热303.15(±0.1) K好的带有0.45 μm滤头的移液枪取上层清液至100 mL的容量瓶中,称重、定容、摇匀、待测。用已经预热好的药勺取下层湿渣于100 mL容量瓶中,称重、溶解、定容、摇匀、待测。将剩余湿渣快速抽滤并用无水乙醇多次淋洗,将滤渣放入干燥箱303.15 (±0.1) K中烘干,研磨后装袋送检XRD分析。实验流程如图 2所示。

|
| 图 1 等温溶解平衡实验装置 Fig.1 Experimental setup for isothermal dissolution equilibrium |
| |

|
| 图 2 相平衡实验流程图 Fig.2 Flow chart of phase equilibrium experiment |
| |
平衡时间确定方法:在体系搅拌、恒温4.0 h后,按照实验方法中的取样分析操作,测定对应离子含量并记录数据;每隔0.5 h取样分析,当测定化学组成不变时认为体系溶解平衡。文献[17, 22]测定了该体系在283.15和353.15 K下的平衡时间分别为8和10 h。本研究以前后2次NH4+、H2PO4-、Cl-含量的相对误差小于0.5%时,认为其化学组成不变达到了溶解平衡。经预实验研究得到该体系在303.15~343.15 K下的溶解平衡时间为8 h。
1.3 分析方法采用甲醛法测定NH4+离子(相对偏差小于0.5%)[34];钼锑抗比色法测定H2PO4-离子(相对偏差小于0.5%)[35];铁铵矾指示剂法测定Cl-的含量(相对偏差小于0.5%)[36];差减法计算K+的含量;湿渣法结合X射线粉末衍射法测定平衡固相组成[37]。实验中每个样本至少分析3次,并取平均值。
2 结果与讨论 2.1 三元体系NH4H2PO4-NH4Cl-H2O溶解度数据 2.1.1 可靠性实验为验证实验装置、试验方法及分析方法的可行性,前期测定了纯NH4Cl和NH4H2PO4在303.15、323.15、333.15和343.15 K下水中的溶解度。将其与文献值[38]进行比较,结果列于表 1。
| T/K | 物质 | 溶解度S/% | 相对误差/% | |
| 实验值 | 文献值 | |||
| 303.15 | NH4Cl | 29.04 | 29.28 | 0.83 |
| NH4H2PO4 | 30.24 | 30.51 | 0.89 | |
| 323.15 | NH4Cl | 33.38 | 33.51 | 0.39 |
| NH4H2PO4 | 40.99 | 40.83 | 0.39 | |
| 333.15 | NH4Cl | 34.21 | 34.30 | 0.26 |
| NH4H2PO4 | 45.28 | 45.21 | 0.15 | |
| 343.15 | NH4Cl | 37.44 | 37.58 | 0.37 |
| NH4H2PO4 | 49.40 | 49.65 | 0.51 | |
由表 1可知,采用该方法测得的纯NH4Cl和NH4H2PO4溶解度与文献值的相对误差小于1%,说明本实验所采用的实验方法、分析方法及仪器设备是适用的,故后续实验确定的实验最大误差不允许超过1%。
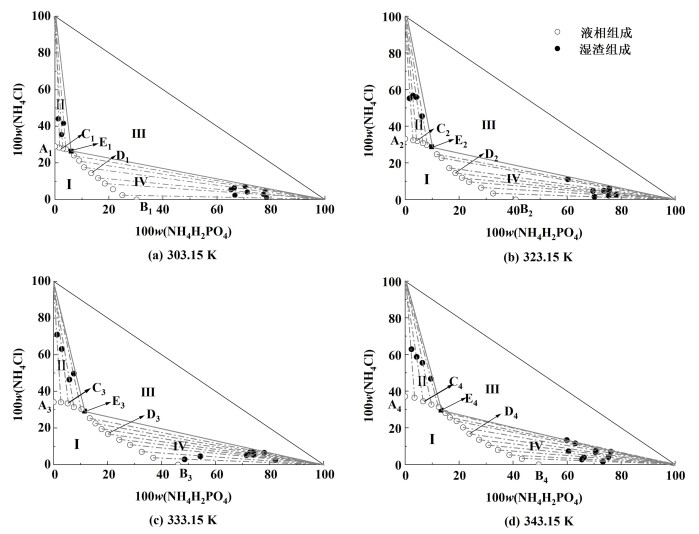
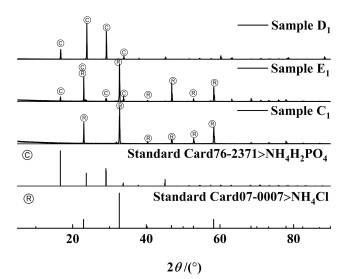
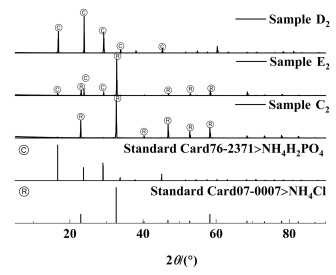
2.1.2 不同温度下的溶解度数据及相图三元体系NH4H2PO4-NH4Cl-H2O在303.15、323.15、333.15和343.15 K下的溶解度数据见表 2,并绘制了各个温度下的等温溶解度相图,如图 3所示。同时结合文献[17, 22, 32]绘制了283.15~353.15 K范围的溶解度曲线对比图,如图 4所示。为了对不同区域固相组成进一步鉴定,本研究用X射线衍射法对图 3中303.15 K下点C1、E1、D1,323.15 K下的点C2、E2、D2,333.15 K下的点C3、E3、D3,343.15 K下的点C4、E4、D4固相进行了鉴定。其XRD图分别见图 5、图 6、图 7和图 8。
| T/K | 编号 | 平衡固相 | 液相质量分数w/% | 固相质量分数w/% | Pitzer-Harvie-Weare模型计算液相质量分数w/% | |||||
| NH4H2PO4 | NH4Cl | NH4H2PO4 | NH4Cl | NH4H2PO4 | NH4Cl | |||||
| 303.15 | 1(B1) | P | 30.24 | 0.00 | 28.91 | 0.00 | ||||
| 2 | P | 24.93 | 2.45 | 1.06 | 20.63 | 26.70 | 2.56 | |||
| 3 | P | 21.47 | 5.65 | 2.39 | 30.94 | 21.99 | 5.83 | |||
| 4 | P | 18.51 | 8.77 | 3.04 | 19.72 | 18.24 | 8.77 | |||
| 5 | P | 16.03 | 11.70 | 4.22 | 24.57 | 15.31 | 11.35 | |||
| 6 | P | 13.41 | 14.46 | 5.46 | 29.30 | 13.20 | 14.49 | |||
| 7 | P | 10.84 | 17.63 | 6.44 | 27.16 | 11.05 | 18.27 | |||
| 8 | P | 8.96 | 21.30 | 5.18 | 16.93 | 8.69 | 20.73 | |||
| 9 | P | 6.57 | 25.93 | 8.17 | 21.36 | 6.43 | 24.83 | |||
| 10(E1) | C+P | 6.01 | 26.29 | 14.50 | 53.56 | 6.39 | 26.30 | |||
| 11 | C | 4.04 | 27.47 | 41.60 | 55.20 | 4.05 | 27.54 | |||
| 12 | C | 3.01 | 27.93 | 35.58 | 61.99 | 2.99 | 27.83 | |||
| 13 | C | 1.53 | 28.41 | 44.09 | 54.82 | 1.53 | 28.37 | |||
| 14(A1) | C | 0.00 | 29.04 | 0.00 | 29.10 | |||||
| RAD | 2.29 | |||||||||
| RMSD | 0.54 | |||||||||
| 323.15 | 1(B2) | P | 40.99 | 0.00 | 36.05 | 0.00 | ||||
| 2 | P | 32.55 | 3.31 | 1.57 | 28.31 | 35.07 | 3.28 | |||
| 3 | P | 28.16 | 6.48 | 2.23 | 22.36 | 30.82 | 6.40 | |||
| 4 | P | 23.66 | 9.60 | 2.64 | 19.17 | 26.05 | 9.56 | |||
| 5 | P | 21.08 | 11.97 | 4.70 | 25.77 | 22.36 | 11.96 | |||
| 6 | P | 18.67 | 14.50 | 4.79 | 21.61 | 18.64 | 14.52 | |||
| 7 | P | 16.37 | 17.16 | 4.87 | 19.60 | 15.11 | 17.22 | |||
| 8 | P | 13.50 | 22.64 | 6.15 | 18.16 | 13.12 | 22.57 | |||
| 9 | P | 11.72 | 24.73 | 11.14 | 28.62 | 11.52 | 24.11 | |||
| 10(E2) | C+P | 9.92 | 28.69 | 17.84 | 37.90 | 9.92 | 28.66 | |||
| 11 | C | 7.97 | 29.92 | 45.48 | 48.23 | 7.97 | 30.12 | |||
| 12 | C | 6.61 | 30.78 | 55.85 | 40.02 | 6.61 | 30.91 | |||
| 13 | C | 4.45 | 32.00 | 56.75 | 40.32 | 4.45 | 31.67 | |||
| 14 | C | 3.00 | 32.41 | 55.20 | 43.17 | 3.00 | 31.76 | |||
| 15(A2) | C | 0 | 33.38 | 0.00 | 33.40 | |||||
| RAD | 2.26 | |||||||||
| RMSD | 1.26 | |||||||||
| 333.15 | 1(B3) | P | 45.28 | 0.00 | 43.50 | 0.00 | ||||
| 2 | P | 36.92 | 3.79 | 48.53 | 2.93 | 40.53 | 3.99 | |||
| 3 | P | 32.68 | 7.04 | 54.31 | 4.65 | 33.85 | 7.24 | |||
| 4 | P | 28.33 | 10.79 | 82.16 | 2.63 | 27.40 | 10.51 | |||
| 5 | P | 24.33 | 13.67 | 71.51 | 5.24 | 23.87 | 13.42 | |||
| 6 | P | 20.11 | 16.82 | 74.01 | 5.37 | 20.57 | 17.10 | |||
| 7 | P | 17.76 | 19.43 | 72.73 | 6.81 | 17.83 | 19.57 | |||
| 13 | C | 5.20 | 33.42 | 2.98 | 63.14 | 4.98 | 32.75 | |||
| 14 | C | 2.70 | 34.07 | 1.25 | 70.92 | 2.73 | 32.93 | |||
| 15(A3) | C | 0.00 | 34.21 | 0.00 | 35.45 | |||||
| RAD | 2.61 | |||||||||
| RMSD | 0.98 | |||||||||
| 8 | P | 15.50 | 22.58 | 73.96 | 6.83 | 14.94 | 22.34 | |||
| 9 | P | 13.35 | 25.38 | 78.04 | 6.50 | 12.92 | 25.71 | |||
| 10(E3) | C+P | 11.53 | 29.15 | 41.59 | 25.57 | 10.55 | 29.41 | |||
| 11 | C | 10.32 | 30.33 | 7.50 | 49.66 | 10.36 | 31.17 | |||
| 12 | C | 7.47 | 31.48 | 5.85 | 46.41 | 7.44 | 31.38 | |||
| 343.15 | 1(B4) | P | 49.40 | 0.00 | 46.76 | 0.00 | ||||
| 2 | P | 43.26 | 3.19 | 73.28 | 1.53 | 43.35 | 2.67 | |||
| 3 | P | 38.64 | 5.21 | 65.44 | 2.73 | 41.34 | 5.24 | |||
| 4 | P | 34.36 | 7.98 | 66.23 | 3.91 | 36.42 | 8.42 | |||
| 5 | P | 30.88 | 10.61 | 75.32 | 3.95 | 31.96 | 11.23 | |||
| 6 | P | 27.36 | 13.52 | 60.51 | 7.23 | 27.58 | 14.23 | |||
| 7 | P | 23.79 | 16.78 | 70.54 | 6.60 | 23.45 | 17.49 | |||
| 8 | P | 21.49 | 20.25 | 70.60 | 7.54 | 19.84 | 20.71 | |||
| 9 | P | 19.04 | 23.70 | 76.17 | 7.06 | 17.21 | 24.32 | |||
| 10 | P | 16.54 | 25.71 | 63.04 | 11.53 | 15.88 | 25.52 | |||
| 11 | P | 14.97 | 28.10 | 59.87 | 13.32 | 14.68 | 27.93 | |||
| 12(E4) | C+P | 13.40 | 29.56 | 57.88 | 20.15 | 13.96 | 29.22 | |||
| 13 | C | 12.44 | 31.44 | 9.52 | 46.71 | 12.40 | 31.82 | |||
| 14 | C | 9.74 | 32.73 | 6.44 | 55.42 | 9.70 | 32.89 | |||
| 15 | C | 6.72 | 34.49 | 4.26 | 58.74 | 6.66 | 34.27 | |||
| 16 | C | 3.49 | 36.52 | 2.34 | 62.93 | 3.43 | 35.37 | |||
| 17(A4) | C | 0.00 | 37.44 | 0.00 | 38.06 | |||||
| RAD | 3.18 | |||||||||
| RMSD | 0.97 | |||||||||
| 注:C为氯化铵;P为磷酸二氢铵;空格为计算值与实验值相同;A(1, 2, 3, 4)表示氯化铵的饱和点;B(1, 2, 3, 4)表示磷酸二氢铵的饱和点;E(1, 2, 3, 4)表示该温度下的共饱和点;T的标准不确定度:u(T)=0.1 K。 | ||||||||||
|
| 图 3 三元体系NH4Cl-NH4H2PO4-H2O在303.15、323.15、333.15和343.15 K时等温溶解相图 Fig.3 Phase diagram of isothermal dissolution of ternary system NH4Cl-HN4H2PO4-H2O at 303.15、323.15、333.15、343.15 K |
| |

|
| 图 4 三元体系NH4Cl-NH4H2PO4-H2O在不同温度下的等温溶解相图 Fig.4 Comparison of phase diagrams of ternary system NH4Cl-NH4H2PO4-H2O at different temperatures |
| |
|
| 图 5 303.15 K下点C1、D1和E1平衡固相的XRD图谱分析 Fig.5 XRD pattern analysis of C1, D1, E1 equilibrium solid phase at 303.15 K |
| |
|
| 图 6 323.15 K下点C2、D2和E2平衡固相的XRD图谱分析 Fig.6 XRD pattern analysis of C2, D2, E2 equilibrium solid phase at 323.15 K |
| |
|
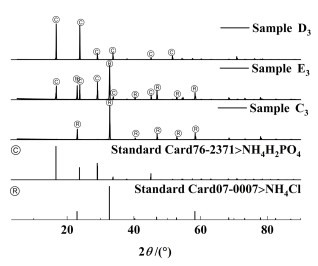
| 图 7 333.15 K下点C3、D3和E3平衡固相的XRD图谱分析 Fig.7 XRD pattern analysis of C3, D3, E3 equilibrium solid phase at 333.15 K |
| |
|
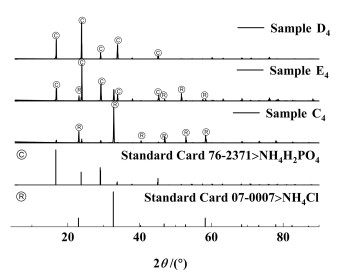
| 图 8 343.15 K下点C4、D4和E4平衡固相的XRD图谱分析 Fig.8 XRD pattern analysis of C4, D4, E4 equilibrium solid phase at 343.15 K |
| |
由表 2和图 3可知,该体系在303.15、323.15、333.15和343.15 K相图趋势一致,均只有1个共饱和点、2条单变量曲线及4个区。点E1、E2、E3、E4为共饱和点;曲线(A1E1、A2E2、A3E3、A4E4)为NH4Cl溶解度曲线;曲线(B1E1、B2E2、B3E3、B4E4)为NH4H2PO4溶解度曲线;2条单变量曲线将相图分为4个区域:Ⅰ区为不饱和区、Ⅱ区为NH4Cl结晶区、Ⅲ区为NH4Cl和NH4H2PO4共结晶区、Ⅳ区为NH4H2PO4结晶区;NH4H2PO4结晶区面积大于NH4Cl结晶区面积,并且NH4H2PO4溶解度从起始点(B)到共饱和点(E)跨度大,表明NH4Cl对NH4H2PO4有强烈的盐析作用,使得NH4H2PO4更容易从混合溶液中析出。
由图 4可知温度从353.15 K下降到283.15 K过程中,该体系不饱和区明显减少,NH4Cl和NH4H2PO4共结晶区变大,共饱和点下移,共饱和点按每100 g水中溶质计算,NH4H2PO4溶解度由60.39 g下降至32.88 g,NH4Cl溶解度由31.07 g下降至6.22 g;因此,随着温度的降低NH4H2PO4、NH4Cl溶解度都降低;同时NH4H2PO4结晶区变大、NH4Cl结晶区变小,说明温度降低更有利于NH4H2PO4结晶。对比各温度下的溶解度曲线变化发现:该体系在该温度范围都没有固溶体或复盐生成,溶解度曲线随温度变化规律一致。因此,温度的变化只改变了溶解度数据,没有改变该体系相图的整体结构。
由图 5、图 6、图 7和图 8可知,C1、C2、C3和C4点处的平衡固相强度峰与NH4Cl(07-0007)标准卡片对应,表明A1、A2、A3和A4点处的平衡固相为NH4Cl;D1、D2、D3和D4点处的平衡固相强度峰与NH4H2PO4(76-2371)标准卡片对应,表明B点处的平衡固相为NH4H2PO4;E1、E2、E3和E4点处的平衡固相强度峰与NH4Cl(07-0007)和NH4H2PO4(76-2371)标准卡片对应,表明E1、E2、E3和E4点为共饱和点且共饱和点处的平衡固相为NH4Cl、NH4H2PO4的简单混合物,没有固溶体、复盐生成;表明该三元体系为简单的共饱和体系。
2.2 溶解度数据关联 2.2.1 Pitzer-Havie-Weare模型Pitzer建立了电解质溶液的离子相互作用热力学模型[39],Harvie和Weare提出了更方便的混合电解质的理论渗透系数和离子活度系数的计算公式[40],即Pitzer-Havie-Weare[式(1)和式(2)]。三元体系NH4H2PO4-NH4Cl-H2O中含有电解质NH4H2PO4和NH4Cl,故可用该模型计算该体系的溶解度数据。
| $ \begin{gathered} \ln \gamma_M=z_M^2 F+\sum\limits_{a=1}^{N_a} m_a\left(2 B_{M a}+Z C_{M a}\right)+ \\ \sum\limits_{c=1}^{N_c} m_c\left(2 {\mathit{\Phi}} _{M c}+\sum\limits_{a=1}^{N_a} m_a \psi_{M c a}\right)+ \\ \sum\limits_{a=1}^{N_a-1} \sum\limits_{a^{\prime}=a+1}^{N_a} m_a m_{a^{\prime}} \psi_{M a a^{\prime}}+\left|z_M\right| \sum\limits_{c=1}^{N_c} \sum\limits_{a=1}^{N_a} m_a m_c C_{c a} \end{gathered} $ | (1) |
| $ \begin{gathered} \ln \gamma_X=z_X^2 F+\sum\limits_{c=1}^{N_c} m_c\left(2 B_{X_c}+Z C_{X_c}\right)+ \\ \sum\limits_{a=1}^{N_a} m_a\left(2 {\mathit{\Phi}}_{X a}+\sum\limits_{c=1}^{N_c} m_c \psi_{X c a}\right)+ \\ \sum\limits_{c=1}^{N_c-1} \sum\limits_{c^{\prime}=c+1}^{N_c} m_c m_{c^{\prime}} \psi_{X c c^{\prime}}+\left|z_M\right| \sum\limits_{c=1}^{N_c} \sum\limits_{a=1}^{N_a} m_a m_c C_{c a} \end{gathered} $ | (2) |
其中F可表示为方程式(3):
| $ \begin{gathered} F=-A^{{\mathit{\Phi}}}\left[I^{1 / 2} /\left(1+1.2 I^{1 / 2}\right)+\right. \\ \left.2 / 1.2 \ln \left(1+1.2 I^{1 / 2}\right)\right]+\sum\limits_{c=1}^{N_c} \sum\limits_{a=1}^{N_a} m_c m_a B_{c a}^{\prime}+ \\ \sum\limits_{c=1}^{N_{c-1}} \sum\limits_{c^{\prime}=c+1}^{N_c} m_c m_{c^{\prime}} {\mathit{\Phi}}_{c c^{\prime}}^{\prime}+\sum\limits_{a=1}^{N_{a-1}} \sum\limits_{a^{\prime}=a+1}^{N_a} m_a m_{a^{\prime}} {\mathit{\Phi}}_{a a^{\prime}}^{\prime} \end{gathered} $ | (3) |
式(1)~(3)中:M、c′、c:阳离子;X、a′、a:阴离子;Nc、Na、Nn:阳离子、阴离子及中性分子的种类数;γ、Z、m:离子的活度系数、离子的价数、离子的质量摩尔浓度;B、B′、Φ、Фij、Φij、Φ′ij:第二维里参数;AΦ:渗透系数的Debye-Hükel的系数;β(0)、β(1)、CΦ、ψ和θ为Pitzer模型的特征参数。
2.2.2 溶解度理论计算在本研究中采用Pitzer-Havie-Weare模型关联计算三元体系NH4H2PO4-NH4Cl-H2O在303.15、323.15、333.15和343.15 K下的溶解度数据。该模型中在各温度下的Debye-Huckel参数AФ根据文献[41]建立的温度关联方程得到,结果见表 3。二元体系NH4Cl-H2O各温度下的单盐参数β(0)、β(1)、CФ根据文献[42]得到的Pitzer参数与温度关联式计算得到,结果见表 4;二元体系NH4H2PO4-H2O各温度下的单盐参数β(0)、β(1)、CФ在文献[43]中已计算得到,结果见表 5。三元体系NH4H2PO4-NH4Cl-H2O中的混合作用参数ФCl-, H2PO4-、ΨNH4+, Cl-, H2PO4-和NH4H2PO4以及NH4Cl在各温度下的平衡常数K通过溶解度数据关联得到,结果见表 6和表 7。利用该模型关联得到的各温度下的溶解度数据见表 2,各温度下的关联计算值与实验值对比见图 9。
| T/K | AФ |
| 303.15 | 0.395 0 |
| 323.15 | 0.410 1 |
| 333.15 | 0.418 6 |
| 343.15 | 0.427 6 |
| T/K | 单盐参数 | ||
| β(0) | β(1) | CФ | |
| 303.15 | 0.053 0 | 0.194 5 | -0.003 0 |
| 323.15 | 0.055 0 | 0.211 6 | -0.003 2 |
| 333.15 | 0.055 2 | 0.221 2 | -0.003 2 |
| 343.15 | 0.055 0 | 0.231 5 | -0.003 1 |
| T/K | 单盐参数 | ||
| β(0) | β(1) | CФ | |
| 303.15 | -0.079 0 | -0.370 5 | 0.008 8 |
| 323.15 | -0.066 1 | -0.445 7 | 0.005 5 |
| 333.15 | -0.061 8 | -0.459 1 | 0.004 5 |
| 343.15 | -0.057 1 | -0.500 2 | 0.003 6 |
| 混合作用参数 | T/K | |||
| 303.15 | 323.15 | 333.15 | 343.15 | |
| ФCl-, H2PO4- | 1.97 | 1.30 | 0.93 | 0.47 |
| ΨNH4+, Cl-, H2PO4- | -0.26 | -0.14 | -0.09 | -0.04 |
| 平衡常数 | T/K | |||
| 303.15 | 323.15 | 333.15 | 343.15 | |
| KNH4Cl | 18.18 | 25.98 | 27.90 | 33.10 |
| KNH4H2PO4 | 0.83 | 1.28 | 1.63 | 1.75 |

|
| 图 9 三元体系NH4H2PO4-NH4Cl-H2O Pitzer-Havie-Weare模型计算值与实验溶解度数据对比 Fig.9 Comparison of calculated values of the ternary system NH4H2PO4-NH4Cl-H2O Pitzer-Havie-Weare model and experimental solubility data |
| |
由表 2可以得到Pitzer-Havie-Weare模型关联计算该体系各温度下的溶解度数据的RAD值和RMSD值分别为:2.29和0.54(303.15 K)、2.26和1.26(323.15 K)、2.61和0.98(333.15 K)、3.18和0.97(343.15 K)。由表 2和图 9可知该模型可以用来关联该体系的溶解度数据。
3 结论通过等温溶解平衡法测定了303.15、323.15、343.15和353.15 K时NH4H2PO4-NH4Cl-H2O三元系的溶解度数据并绘制了相图。得出如下结论:该体系在不同温度下均为简单共饱和型,无复盐和固溶体形成,相图有3个结晶区,分别为:NH4H2PO4结晶区、NH4Cl结晶区、NH4H2PO4和NH4Cl共晶区。温度的变化并未改变该三元体系共饱和点的平衡固相组成;随着温度下降,该体系共饱和点下移,不饱和区明显减小,NH4H2PO4和NH4Cl共晶区增大,NH4H2PO4结晶区增大,NH4Cl结晶区减小。因此,温度的下降有更利于NH4H2PO4晶体析出,不利于NH4Cl晶体析出。
采用Pitzer-Harvie-Weare模型对该体系进行了溶解度数据的关联计算。该模型计算值与实验值的RAD最大值3.18,RMSD最大值为0.98,计算结果与实验值基本吻合。
| [1] |
NIU H, PANG Z, FALLAH N, et al. Diversity of microbial communities and soil nutrients in sugarcane rhizosphere soil under water soluble fertilizer[J]. PLoS One, 2021. DOI:10.1371/journal.pone.0245626 |
| [2] |
XIE C, ZHANG T, WANG X. Solid-liquid phase equilibria in aqueous solutions of four common fertilizers at 303.2 K and atmospheric pressure[J]. Fluid Phase Equilibria, 2018, 474: 131-140. DOI:10.1016/j.fluid.2018.07.016 |
| [3] |
SUN G, HU T, LIU X, et al. Optimizing irrigation and fertilization at various growth stages to improve mango yield, fruit quality and water-fertilizer use efficiency in xerothermic regions[J]. Agricultural Water Management, 2022. DOI:10.1016/j.agwat.2021.107296 |
| [4] |
YANG G, ZHAO H, CHEN Q, et al. Potassium chloride-modified urea phosphate with response surface optimization and its application effect on maize in saline-alkali soil[J]. ACS Omega, 2020, 5(28): 17255-17265. DOI:10.1021/acsomega.0c01428 |
| [5] |
PAWAR N, SAHA A, NANDAN N, et al. Solution cocrystallization: A scalable approach for cocrystal production[J]. Crystals, 2021. DOI:10.3390/cryst11030303 |
| [6] |
GUI L, YANG H, HUANG H. Liquid solid fluidized bed crystallization granulation technology: Development, applications, properties, and prospects[J]. Journal of Water Process Engineering, 2022. DOI:10.1016/j.jwpe.2021.102513 |
| [7] |
GAGNIERE E, MANGIN D, PUEL F, et al. Formation of co-crystals: Kinetic and thermodynamic aspects[J]. Journal of Crystal Growth, 2009, 311(9): 2689-2695. DOI:10.1016/j.jcrysgro.2009.02.040 |
| [8] |
YANG H Y, RASMUSON A C. Phase equilibrium and mechanisms of crystallization in liquid-liquid phase separating system[J]. Fluid Phase Equilibria, 2015, 385: 120-128. DOI:10.1016/j.fluid.2014.11.007 |
| [9] |
WALAS S M. Phase Equilibria in Chemical Engineering[M]. Britain: Butterworth-Heinemann, 1985.
|
| [10] |
蒋成君, 程桂林. 共结晶分离技术研究进展[J]. 化工进展, 2020, 39(1): 311-319. JIANG Chengjun, CHENG Guilin. Progress in co-crystallization as a separation technology[J]. Chemical Industry and Engineering Progress, 2020, 39(1): 311-319. DOI:10.16085/j.issn.1000-6613.2019-0620 (in Chinese) |
| [11] |
KHAN H I. Appraisal of biofertilizers in rice: To supplement inorganic chemical fertilizer[J]. Rice Science, 2018, 25(6): 357-362. DOI:10.1016/j.rsci.2018.10.006 |
| [12] |
ASSIMI T E, BENIAZZA R, RAIHANE M, et al. Overview on progress in polysaccharides and aliphatic polyesters as coating of water-soluble fertilizers[J]. Journal of Coatings Technology and Research, 2022, 19(4): 989-1007. DOI:10.1007/s11998-022-00613-1 |
| [13] |
胡雪, 朱静, 王睿哲, 等. (NH2)2CO-NH4H2PO4-H2O三元系10 ℃相平衡研究[J]. 无机盐工业, 2019, 51(5): 41-44. HU Xue, ZHU Jing, WANG Ruizhe, et al. Research on phase equilibrium of ternary system of(NH2)2CO-NH4H2PO4-H2O at 10 ℃[J]. Inorganic Chemicals Industry, 2019, 51(5): 41-44. (in Chinese) |
| [14] |
胡雪. 常压下(NH2)2CO-NH4H2PO4-KCl-H2O在283.15 K的相平衡研究[D]. 贵阳: 贵州大学, 2019 HU Xue. Phase equilibrium study of (NH2)2CO-NH4H2PO4-KCl-H2O at 283.15 K under atmospheric pressure[D]. Guiyang: Guizhou University, 2019(in Chinese) |
| [15] |
杨家敏, 朱静, 胡雪, 等. 283.15 K下三元KCl-NH4Cl-H2O和KH2PO4-NH4H2PO4-H2O体系固-液相平衡测定与关联[J]. 无机盐工业, 2021, 53(1): 30-35. YANG Jiamin, ZHU Jing, HU Xue, et al. Determination and correlation for solid-liquid phase equilibrium of ternary KCl-NH4Cl-H2O and KH2PO4-NH4H2PO4-H2O systems at 283.15 K[J]. Inorganic Chemicals Industry, 2021, 53(1): 30-35. (in Chinese) |
| [16] |
杨家敏. KCl-NH4H2PO4-CO(NH2)2-H2O体系在283.15 K下固-液相平衡研究[D]. 贵阳: 贵州大学, 2020 YANG Jiamin. Research on the solid-liquid phase equilibrium of KCl-NH4H2PO4-CO(NH2)2-H2O system at 283.15 K[D]. Guiyang: Guizhou University, 2020 (in Chinese) |
| [17] |
吴强, 胡雪, 朱静, 等. KH2PO4-KCl-H2O、NH4H2PO4-NH4Cl-H2O三元体系283.15 K相平衡研究[J]. 无机盐工业, 2020, 52(11): 24-28. WU Qiang, HU Xue, ZHU Jing, et al. Phase equilibrium of KH2PO4-KCl-H2O and NH4H2PO4-NH4Cl-H2O ternary system at 283.15 K[J]. Inorganic Chemicals Industry, 2020, 52(11): 24-28. DOI:10.11962/1006-4990.2019-0639 (in Chinese) |
| [18] |
邓文清. K+, NH4+//Cl-, H2PO4-, (NH2)2CO-H2O在313.15 K下固—液相平衡研究[D]. 贵阳: 贵州大学, 2022 DENG Wenqing. Solid-liquid phase equilibrium study of K+, NH4+//Cl-, H2PO4-, (NH2)2CO-H2O at 313.15 K[D]. Guiyang: Guizhou University, 2022(in Chinese) |
| [19] |
樊小娟, 朱静, 邓文清, 等. 交互四元体系K+, NH4+//Cl-, H2PO4--H2O在313.15 K时相平衡研究[J]. 无机盐工业, 2022, 54(10): 102-108. FAN Xiaojuan, ZHU Jing, DENG Wenqing, et al. Study on phase equilibria of reciprocal quaternary system of K+, NH4+//Cl-, H2PO4--H2O at 313.15 K[J]. Inorganic Chemicals Industry, 2022, 54(10): 102-108. (in Chinese) |
| [20] |
王肖丽, 朱静, 吴强, 等. NH4+, K+//H2PO4-, CO(NH2)2-H2O四元体系298.15 K相平衡研究[J]. 化学工程, 2021, 49(8): 39-44. WANG Xiaoli, ZHU Jing, WU Qiang, et al. Study on quaternary phase equilibrium of NH4+, K+//H2PO4-, CO(NH2)2-H2O system at 298.15 K[J]. Chemical Engineering(China), 2021, 49(8): 39-44. DOI:10.3969/j.issn.1005-9954.2021.08.008 (in Chinese) |
| [21] |
黄林川, 李天祥, 杨家敏, 等. 三元体系KH2PO4-CO(NH2)2-H2O在283.15 K的固液相平衡测定与关联[J]. 化学工业与工程, 2021, 38(3): 64-69. HUANG Linchuan, LI Tianxiang, YANG Jiamin, et al. Determination and correlation of solid-liquid equilibrium of ternary system KH2PO4-CO(NH2)2-H2O at 283.15 K[J]. Chemical Industry and Engineering, 2021, 38(3): 64-69. (in Chinese) |
| [22] |
黄林川. KCl-NH4H2PO4-(NH2)2CO-H2O体系在353.15 K下的相平衡研究[D]. 贵阳: 贵州大学, 2021 HUANG Linchuan. Study on phase equilibrium of KCl-NH4H2PO4-(NH2)2CO-H2O system at 353.15 K[D]. Guiyang: Guizhou University, 2021 (in Chinese) |
| [23] |
陈艳, 李天祥, 王肖丽, 等. 353.15 K下三元体系KCl-KH2PO4-H2O和KH2PO4-CO(NH2)2-H2O固液相平衡测定与关联[J]. 化学工程, 2022, 50(6): 43-49. CHEN Yan, LI Tianxiang, WANG Xiaoli, et al. Determination and correlation of solid-liquid equilibria for ternary systems KCl-KH2PO4-H2O and KH2PO4-CO(NH2)2-H2O at 353.15 K[J]. Chemical Engineering (China), 2022, 50(6): 43-49. (in Chinese) |
| [24] |
张逢星, 魏小兰, 崔斌, 等. N—P—K复肥四元体系KCl-KH2PO4-CO(NH2)2-H2O在298.2 K相图[J]. 盐湖研究, 1996, 4(1): 59-62. ZHANG Fengxing, WEI Xiaolan, CUI Bin, et al. Phase diagram of the quaternary system KCI-KH2PO4-CO (NH2)2-H2O at 298.2 K[J]. Journal of Salt Lake Research, 1996, 4(1): 59-62. (in Chinese) |
| [25] |
YU X, ZENG Y, YAO H, et al. Metastable phase equilibria in the aqueous ternary systems KCl + MgCl2 + H2O and KCl + RbCl + H2O at 298.15 K[J]. Journal of Chemical & Engineering Data, 2011, 56(8): 3384-3391. |
| [26] |
赵长伟, 马沛生, 郭瓦力, 等. KCl-NH4Cl-H2O三元水盐体系溶解度的研究[J]. 化学工业与工程, 2003, 20(3): 145-149. ZHAO Changwei, MA Peisheng, GUO Wali, et al. Study on the solubility of the three-component system KCl-NH4Cl-H2O[J]. Chemical Industry and Engineering, 2003, 20(3): 145-149. (in Chinese) |
| [27] |
ZHAO B, GENG G, CHEN J, et al. The ternary system phase equilibrium of KCl-NH4Cl-H2O at 80℃[J]. Advanced Materials Research, 2013, 834/835/836: 519-522. |
| [28] |
SHEN W, REN Y, ZHANG X, et al. Solid-liquid phase equilibrium for the ternary system (potassium chloride+potassium dihydrogen phosphate+water) at (298.15 and 313.15) K[J]. Journal of Chemical & Engineering Data, 2015, 60(7): 2070-2078. |
| [29] |
ZHANG Y, YU M, LIU J, et al. Solid-liquid equilibrium, structural features and separation process of ammonium potassium dihydrogen phosphate solid solution[J]. Chemical Physics, 2021. DOI:10.1016/j.chemphys.2021.111109 |
| [30] |
SILCOCK E. Solubility of inorganic and organic compounds V3: Ternary and multicomponent systems of inorganic substances[M]. Britain: Pergamon, 1979.
|
| [31] |
EL HANTATI S, NOUR Z, DINANE A, et al. Thermodynamic properties data of ternary system NH4Cl-NH4H2PO4-H2O at 298.15 K including the solubility data[J]. Journal of Molecular Liquids, 2022. DOI:10.1016/j.molliq.2022.119381 |
| [32] |
何婷婷. Na+, NH4+//Cl-, H2PO4-, SO42--H2O子体系稳定相平衡研究[D]. 银川: 宁夏大学, 2018 HE Tingting. Research on stable equilibria in quinary system Na+, NH4+//Cl-, H2PO4-, SO42--H2O and its sub-system[D]. Yinchuan: Ningxia University, 2018 (in Chinese) |
| [33] |
邓天龙, 周桓, 陈侠. 水盐体系相图及应用[M]. 北京: 化学工业出版社, 2013.
|
| [34] |
袁砚, 郭永福, 吴伟, 等. 废水中低浓度氨氮的甲醛法快速测定[J]. 工业水处理, 2014, 34(10): 73-75. YUAN Yan, GUO Yongfu, WU Wei, et al. Rapid determination of low-concentration ammonia nitrogen in wastewater by formaldehyde method[J]. Industrial Water Treatment, 2014, 34(10): 73-75. (in Chinese) |
| [35] |
严一乾. 钼锑抗分光光度法测定水中总磷的影响因素分析[J]. 绿色科技, 2017(2): 32-33, 38. YAN Yiqian. Analysis of influencing factors on determination of total phosphorus in water by molybdenum antimony spectrophotometry[J]. Journal of Green Science and Technology, 2017(2): 32-33, 38. (in Chinese) |
| [36] |
辛欣. 硫氰酸铵容量法测定氯离子含量方法的改进研究[J]. 化工管理, 2017(27): 37-39. XIN Xin. Improvement of ammonium thiocyanate volumetric method for determination of chloride ion content[J]. Chemical Enterprise Management, 2017(27): 37-39. (in Chinese) |
| [37] |
FAN X, LI T, WANG X, et al. Measurement and correlation of solid-liquid equilibrium of ternary system KCl-CO(NH2)2-H2O at 313.15 K and 353.15 K[J]. Journal of Molecular Liquids, 2022. DOI:10.1016/j.molliq.2022.119951 |
| [38] |
刘光启, 马连湘, 项曙光. 化学化工特性数据手册.无机卷[M]. 北京: 化学工业出版社, 2002. LIU Guangqi, MA Lianxiang, XIANG Shuguang. Chemical Properties Data Book. Inorganic Volume[M]. BeiJing: Chemical Industry Press, 2002. (in Chinese) |
| [39] |
PITZER K S, MAYORGA G. Thermodynamics of electrolytes. Ⅱ. Activity and osmotic coefficients for strong electrolytes with one or both ions univalent[J]. The Journal of Physical Chemistry, 1973, 77(19): 2300-2308. |
| [40] |
HARVIE C, EUGSTER H, WEARE J. Mineral equilibria in the six-component seawater system, Na-K-Mg-Ca-SO4-Cl-H2O at 25 ℃. Ⅱ: Compositions of the saturated solutions[J]. Geochimica et Cosmochimica Acta, 1982, 46(9): 1603-1618. |
| [41] |
MØLLER N. The prediction of mineral solubilities in natural waters: A chemical equilibrium model for the Na-Ca-Cl-SO4-H2O system, to high temperature and concentration[J]. Geochimica et Cosmochimica Acta, 1988, 52(4): 821-837. |
| [42] |
JI X, LU X, ZHANG L, et al. A further study of solid-liquid equilibrium for the NaCl-NH4Cl-H2O system[J]. Chemical Engineering Science, 2000, 55(21): 4993-5001. |
| [43] |
EL GUENDOUZI M, BENBIYI A. Study of di-hydrogen (Na; K or NH4) orthophosphates in aqueous solutions at temperatures from 298.15 K to 353.15 K[J]. Fluid Phase Equilibria, 2016, 408: 223-231. |
 2023, Vol. 40
2023, Vol. 40