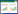2. 清华大学材料学院, 北京 100084
2. School of Materials, Tsinghua University, Beijing 100084, China
石墨烯是由碳原子经sp2杂化构成的单层六角形结构的二维材料[1]。2004年英国曼彻斯特大学科学家Geim和Novoselov用胶带剥离的方法制备出自然条件下可以稳定存在的石墨烯,引起了科学界的广泛关注[2-3],他们因此获得了诺贝尔物理学奖。石墨烯具有优秀的导电性和高载流子迁移率[约为2×105 cm2 ·(V ·s)-1)][4]、较大的电化学窗口、卓越的机械强度、良好的导热性和超大的比表面积等优良特性[5-8]。基于以上性质,石墨烯在储能、电子、环境和催化等领域有广泛的应用前景[9-13]。
常见的制备石墨烯的方法包括:微机械剥离法[2, 14-16]、化学气相沉积法[17-20]、液相剥离法[21-25]、外延生长法[26-29]和化学氧化还原法[30-34]等。这些方法在制备石墨烯方面有着各自的优点,但也存在一定的局限性。
如化学气相沉积法是制备大面积单层石墨烯薄膜的重要方法。但制备过程需要高温及苛刻的条件与设备,成本较高[35-36]。化学氧化还原法虽然能得到较高产率的氧化石墨烯,但是制备过程需要消耗大量的强酸和强氧化剂,工艺复杂,生产的氧化石墨烯存在较多的缺陷。相对而言,电化学法制备石墨烯因具有装置简单、环境友好等优点[37-39],成为很有前景的石墨烯制备方法。
近年来,电化学方法在石墨烯的制备和应用上取得了新的进展,将其作为电极材料应用于锂离子电池和超级电容器等储能领域中,展现出极大的潜力。本论文重点介绍了电化学制备石墨烯的方法、原理及生产石墨烯的质量等,并对石墨烯在储能领域的应用进行综述。
1 电化学方法制备石墨烯电化学制备石墨烯的方法主要包括阳极插层剥离法、阴极还原剥离法、电化学还原法和电泳沉积法等。这几种方法都是基于电化学反应的基本原理,每种方法都有各自的优点和局限性。典型的反应装置通常以石墨作为工作电极,以石墨、铂丝等不同材料作为对电极,以水溶液(如酸、碱或硫酸盐溶液)或非水溶液(有机溶液或离子液体等)作为电解液,以1台能提供恒定电压的直流电源或电化学工作站作为电源[40]。
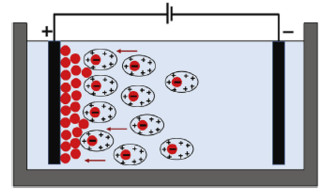
1.1 阳极插层剥离法制备石墨烯阳极插层剥离法是一种最常用的电化学制石墨烯的方法。由于具有较高的剥离效率,较好的剥离效果而被广泛研究[41]。在外加电压作用下,电解液解离出的阴离子向阳极移动,进而插入石墨层间,降低了石墨层间的范德华力,电解过程中水和阴离子分解产生气体促进石墨膨胀、剥离成片状单层或少层石墨烯(图 1)[38]。
Su等[42]用一定量的硫酸做电解液,首先外加1 V的恒定电压,电解5~10 min,然后将电压调到10 V电解1 min。低电压的作用是润湿电极并将硫酸根离子插入到石墨的晶粒边界。剥离产物重新分散到二甲基甲酰胺(DMF)溶液中,有较好的稳定性。经原子力显微镜测试,最终获得的少层石墨烯,65%以上的片层厚度小于2 nm,平均横向尺寸在1~40 μm之间。明显大于通过超声进行液相剥离得到的石墨烯片尺寸[43-44]。使用硫酸做电解液进行电化学剥离,有高效快速的优点,但是生产的石墨烯缺陷密度较高,这是因为硫酸本身对石墨具有较强的氧化性。通过向硫酸溶液中加入一定量的KOH调节电解液的pH值可以获得较高质量的石墨烯薄膜[42]。
最近,有研究者提出两步电化学剥离制备石墨烯的方法[39, 45]。Cao等[45]采用先将石墨纸在浓硫酸电解液中用2.2 V电压电解20 min,形成一阶H2SO4-石墨层间化合物。随后,将其移入0.1 mol/L的硫酸铵电解液中,在10 V电压下,剥离5~10 min,最终收集到的剥离产物的产率高达71%。经TEM测试,得到的石墨烯微片的平均横向尺寸在2~3 μm。AFM测试电化学剥离石墨烯的厚度在1 nm左右,和文献报道的单层氧化石墨烯一致[46-47]。
阳极插层剥离法制得的石墨烯要比采用化学方法制得的氧化石墨烯氧化程度低。因为电化学剥离制得的石墨烯有更少的含氧官能团(C=O,—COO—),碳环结构更加完整[46]。
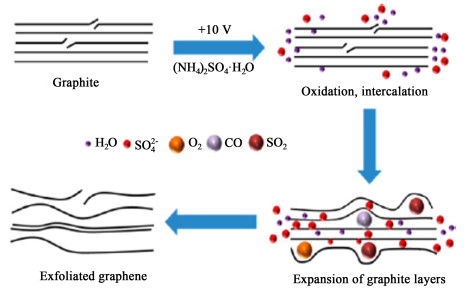
Pei等[39]先对商用石墨纸在+1.6 V较低电压下用浓硫酸进行插层[图 2a)],20 min后可观察到石墨纸颜色变蓝[图 2b)~图 2c)],表明形成了石墨层间化合物,随后将它移入50%稀硫酸中用高电压(+5.0 V)进行剥离,30 s后可看到蓝色的石墨层间化合物变为黄色[图 2d)],剥离形成电化学氧化石墨烯(EGO)的产率高达96%,极大地提高了剥离效率和石墨烯的质量,为后面石墨烯的功能化应用提供了很大帮助。
利用制备出的电化学氧化石墨烯进一步合成了透明导电薄膜,柔韧的石墨烯纸和超轻气凝胶(图 3)。其中透明导电薄膜经HI还原后,透明度为80%,表面电阻约为1.5 kΩ/sq,与通过加热还原氧化石墨烯相似[48]。合成的石墨烯纸的机械强度为175 MPa,高于通过热还原氧化石墨烯制备的石墨烯纸[49]。制备的气凝胶具有高度发达的孔结构和高弹性,它能支撑起自身1 000倍以上的重量而保持结构完整,并能在撤回压力时迅速恢复原状。通过以上方法制备的石墨烯在能源、环境和生物医药等众多领域有潜在的应用价值。
直接用石墨纸或石墨棒作为工作电极,进行阳极插层剥离时,随着插层的进行石墨电极往往会瓦解,掉落下来的石墨将不再参与插层过程,从而导致石墨烯产率较低。为了解决这个问题,Achee等[50]将鳞片石墨放入透析袋中制成了可伸缩的工作电极,在硫酸铵电解液中进行插层剥离。最终获得了较高产率(65%)和较大横向尺寸(>30 μm)的石墨烯片,并提出了石墨电极连续进行电化学插层剥离的方案。
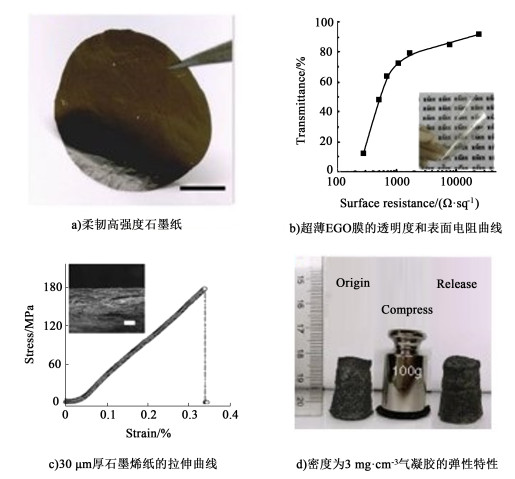
1.2 阴极还原剥离法制备石墨烯阴极还原剥离法制备石墨烯的原理与阳极插层剥离法类似,两者都是在电场力的作用下,使电解液中的离子发生定向移动,克服石墨层间作用力,并与电解产生的气体共插层进入石墨层间,使石墨剥离产生单层或少层石墨烯。两者的主要区别是插入石墨的离子和使石墨剥离的气体不同。图 4是阳极插层剥离法和阴极还原剥离法的反应机理对比图[40]。阳极氧化时,电解液解离出的阴离子插入石墨层间,同时阳极产生的氧气促使石墨膨胀剥离;与此对应,阴极还原时,电解液解离出的阳离子插入石墨层间,阴极产生的氢气使石墨剥离产生单层或少层石墨烯。阴极还原法可以防止石墨烯被过度氧化,并保持sp2碳环结构的完整性。
受锂离子电池中碳酸丙烯酯(PC)会破坏石墨负极的启发,Wang等[51]将这种剥离机理应用于石墨烯的制备,期望得到高质量少层石墨烯片。他们用石墨做电解池阴极,以Li+/PC做电解液,采用高电势[(-15±5) V]来激发Li+/PC共插层进入石墨中。经电化学剥离和超声分散后,最终得到少层石墨烯的平均横向尺寸为1~2 μm,原子力显微镜表征结果显示,大约50%的少层石墨烯层数在2~3层。拉曼光谱分析显示该少层石墨烯的ID/IG小于0.1,表明与氧化石墨烯相比有更低的缺陷密度[52]。另外,研究还发现了电解过程中Li+存在的重要性,电解液中Li+不足或缺少Li+,将导致剥离产率明显下降,由原来的70%降为1%。这表明Li+有着破坏石墨层间作用力并促进其他离子共插层的重要作用。
与阳极氧化法相比,阴极还原法所用的电解液大多是有机溶液而不是水溶液,如十二烷硫酸钠、碳酸丙烯酯、DMF和NMP等[53]。此类有机溶液用于制备石墨烯所起的作用与Li+不同,反应过程中表面活性剂等可以吸附在石墨烯片的表面,会抑制电化学过程中刚剥离的石墨烯重新团聚在一起[51],极大地改善溶液的分散性,但是,也有报道显示,表面活性剂的使用会降低产物的电导率,空穴迁移率等电学性能[54]。
以超声波辅助进行电化学阴极放电的方法,制备出了尺寸较大,层数在2~7层的少层石墨烯[55]。此方法以KOH和(NH4)2SO4的混合碱性溶液为电解液,以高纯石墨棒做该装置的阴阳两极,而阴极石墨棒顶端被削尖以提高其电流密度,并用超声波清洗机辅助剥离[图 5a)]。电解过程中,阴极石墨尖端放置在距电解液1 mm处,阳极石墨棒浸入电解液中,在较高的直流电压下(60 V)阴极发生等离子体放电。水的电解产生的氢气和等离子体混合,加速石墨棒的插层,并在超声波的作用下快速剥离。通过拉曼光谱分析发现[图 5b)],与高纯石墨棒(HG)相比,超声辅助电化学剥离方法得到的石墨烯(UPEEG)的光谱出现红移,且2D峰的强度明显增大,表明剥离产生了石墨烯。图 5c)所示的剥离产物的TEM照片可以明显看到层状结构,表明超声波辅助阴极剥离得到了少层石墨烯。
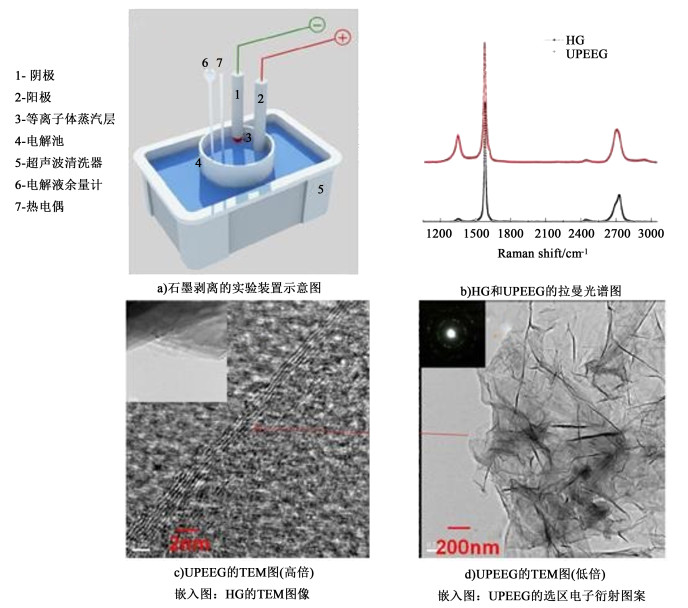
|
| 图 5 石墨剥离的实验装置示意图a)、HG和UPEEG的拉曼光谱图b)、UPEEG的TEM图及嵌入图c)、HG的TEM图像d)[55] Fig.5 Schematic representation of the experimental setup for exfoliation of graphite) a); Raman spectra of HG and UPEEG b); High c) and low d) magnification TEM images of UPEEG.Inset in TEM image of HG. Inset in SAED pattern of UPEEG[55] |
| |
电化学还原法与阴极还原法不同,电化学还原法不需要对石墨进行电化学插层和剥离,而是直接对用化学法得到的氧化石墨烯进行还原。
氧化石墨烯含有较多的含氧官能团,通过电化学还原可降低其氧含量,增加它的导电性。Toh等[56]用滴涂了一定量氧化石墨烯的玻碳电极做工作电极,在1 mol ·L-1的KOH电解液中,以-0.6~-1.5 V vs. Ag/AgCl的电势范围内进行电化学还原,得到电化学还原氧化石墨烯。进一步研究表明,在碱性介质中,进行了电化学还原的氧化石墨烯由于电导率的提高,能增强氧化还原反应的电催化活性,有利于反应的进行。
Liu等[57]采用电化学沉积的方法,在150 V电势下将氧化石墨烯修饰在ITO导电玻璃上制成氧化石墨烯膜。然后,将它作为工作电极,玻碳电极做对电极,在0.1 mol ·L-1的氯化钾溶液中,以10 mV/s的速率进行0 ~-1 V的扫描,最终得到还原氧化石墨烯薄膜。经红外和拉曼光谱分析表明,电化学还原后石墨烯膜的大部分含氧官能团被消除,ID/IG也明显降低。
相比于热还原和化学还原方法,电化学还原法具有绿色、安全、低能耗等优势,近年来有很多报道[57]。
1.4 其他电化学方法电泳沉积技术(EPD)通常在双电极电解池中进行,它的外加电场可以是直流电模式也可以是调制电流模式(如图 6)[61]。石墨烯材料的电泳沉积原理包括电泳和沉积2个部分[62]。电泳过程指当电场作用于氧化石墨烯悬浮液时,带电氧化石墨烯薄片在电场力的驱动下向相反方向移动,沉积过程是在电场力下氧化石墨烯薄片堆积在电极表面。
电泳沉积所得石墨烯膜受氧化石墨烯浓度、施加直流电压和沉积时间的影响,通过调节以上几个因素可让沉积石墨烯膜的厚度从几百纳米到几十微米宽的范围内变化[63]。An等[64]用Hummers法制得的氧化石墨烯分散在水中制得悬浮液,在10 V直流电压下电解1~10 min,风干后得到还原度较高的石墨烯薄膜。在直流电压作用下,氧化石墨烯在电极表面吸附,与电极发生离子交换,被还原为石墨烯。氧化石墨烯和还原氧化石墨烯是用作电泳沉积的主要前驱体,因为存在大量的含氧官能团,易于制备石墨烯分散体,电化学还原发生在电泳沉积过程和电泳沉积过程之后的还原[65-66]。通过分离过程,沉积的石墨烯膜可以成为一个独立的柔性薄膜。采用化学和电化学方法分离出沉积的石墨烯薄膜,获得了大面积、导电性好的还原氧化石墨烯独立膜[66]。
最近的相关报道,表明电泳沉积技术也可用于制备石墨烯/金属基纳米材料[67-69]和石墨烯/高分子聚合物复合材料[70-71]。在石墨烯的加工制备和石墨烯基材料的应用研究领域,人们对电泳沉积技术的研究兴趣日益浓厚,电泳沉积技术逐渐成为一种很有前景的石墨烯制备技术。
2 石墨烯在储能领域的应用 2.1 石墨烯在锂离子电池中的应用锂离子电池因具有高工作电压、高能量密度和低自放电速度,近几年得到了快速的发展[10]。石墨烯具有良好的导电性[载流子迁移率约为2×105 cm2 ·(V ·s)-1,锂离子迁移率为10-7~10-6 S ·cm-1],将其应用于锂离子电池负极材料中,它特殊的网状结构,能为电子传导和离子扩散提供路径,提高电极活性材料的嵌锂性能,进而提高电极材料的导电性和电化学性能[72-74]。但是由纯石墨烯组成的锂离子电池负极材料具有首次库伦效率低、充放电平台较高等缺点,使其不能代替石墨直接做负极材料使用[75]。主要原因是将纯石墨烯作为锂离子电池负极材料时,由于石墨烯之间的团聚重新形成了π-π堆积的石墨结构,从而严重影响锂离子的脱嵌。因此,研究将石墨烯与其他碳材料(如碳纳米管、富勒烯等)和硅基、锡基等材料的复合[76-79]对石墨烯团聚的问题有一定的改善,得到性能优良的锂离子电池负极材料。
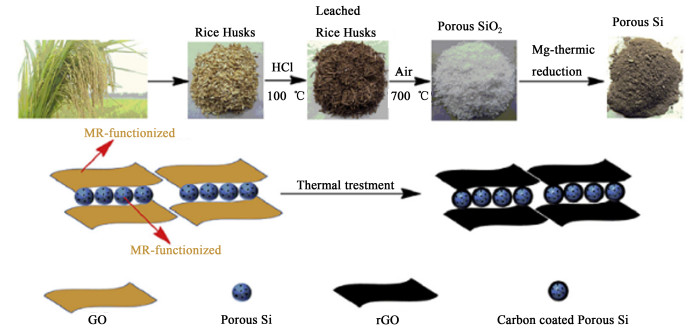
Jiao等[80]用稻谷制备多孔硅,然后与改性氧化石墨烯复合,通过缩合反应和热处理,制备出石墨烯/多孔硅复合材料(图 7),进一步研究发现,将其用于锂离子电池负极材料,显示出良好的循环和倍率性能。Liu等[81]制备了一种三明治结构,柔韧性很好的还原氧化石墨烯/纳米硅复合薄膜。将其用于锂离子电池负极材料时,内部的空隙和非晶态硅纳米薄膜的力学特性可以缓冲嵌锂/脱锂以防止粉化,从而延长循环寿命。这种纳米结构中交替排列的还原氧化石墨烯层可以促进电子的传输,适应硅层体积的变化,并能防止它们团聚。Tian等[82]通过一步水热法合成了一种新颖的三维MoS2/石墨烯纳米线复合材料。其中石墨烯纳米线在MoS2层间充当导电桥梁的作用,能有效促进电子和离子在三维复合结构中的传输。当作为锂离子电池负极材料时,在200 mAh ·g-1的电流密度下,首次放电充电的容量为1 261.5和953.4 mAh ·g-1,经80次循环后,该复合材料仍然有1 009.4 mAh ·g-1的可逆容量,相对于首次循环,容量保持率达80%。
阳极插层剥离法制备的石墨烯比通过改性Hummers法制备的氧化石墨烯有更低的氧化程度,更少的含氧官能团,C/O原子比例比氧化石墨烯更高,通过简单的还原就能得到缺陷更少,sp2结构更完整的石墨烯[45]。阳极插层剥离制备的石墨烯纳米片,经还原后具有相对完整的长程有序的六方晶格结构,电导率较高[83],将其用于锂离子电池负极材料比还原氧化石墨烯更有优势。
2.2 石墨烯在超级电容器中的应用超级电容器是目前最重要的电化学储能体系之一。超级电容器按电荷存储机理可分为双电层电容器和赝电容电容器[84]。超级电容器和锂离子电池相比具有功率密度大,充放电效率高,循环寿命长等特点,但其能量密度相对较低,是限制其发展的主要问题。因此,开发出高能量密度的电极材料是超级电容器发展的重要方向。传统的超级电容器碳基材料主要有活性炭、碳纤维和碳凝胶等[85],相对而言,石墨烯因具有超高的电导率和巨大的比表面积,能提供更多的电化学活性位点,更有利于电子的转移,表现出作为超级电容器电极材料的巨大潜力。
将石墨烯用于双电层超级电容器其理论比电容可达550 F ·g-1[86],远高于其他炭材料基超级电容器。然而,在实际应用中石墨烯基超级电容器的实际容量要远低于理论值,主要原因是石墨烯片层间存在较强的范德华力,使其易于团聚,重新堆叠,极大地降低了石墨烯的有效表面积。提高石墨烯基超级电容器电容性能的方法有很多,常用的有活化造孔[87]、组装3D宏观体[88]和添加导电聚合物[89]等方法。Zhu等[87]采用碱活化的方法对石墨烯造孔,制备出多孔石墨烯,将其用于超级电容器电极可极大地提高它的比容量,功率密度高达75 W ·g-1,比商用超级电容器碳电极高出1个数量级;且在电流密度为2.5 A ·g-1下,恒流充放电10 000次后容量保持率达97%,表现出很好的稳定性。Wang等[90]采用葡萄糖和氯化铵作为原料,经高温煅烧,并在铵盐分解产生气体作用下,制备出3D多孔结构的石墨烯。该三维结构石墨烯比表面积高达1 005 cm2 ·g-1,经电化学测试显示,在1 A ·g-1电流密度下比容量高达250 F ·g-1,在100 A ·g-1较高的电流密度下,比容量仍能达到130 F ·g-1。另外,通过向电极材料中引入氧化钴、二氧化锰和氢氧化镍等过渡金属化合物,产生赝电容反应,是提高电极材料电容性能和能量密度的又一方式。Qin等[91]制备了一种自支撑三维管状和层次化多孔石墨烯膜,通过简单的电化学沉积将MnO2负载到石墨烯膜表面,使该材料不仅具有较高的电子和离子传递速率,而且MnO2在整个电极的质量负荷高达90.5%。电化学测试显示,在5 mV ·s-1的扫描速率下,该电极的比容量达403 F ·g-1。在2 V电势下工作时,能得到超高的体积能量密度28.2 mWh ·cm-3和良好的循环稳定性。Yu等[92]报道了NiCo2O4与石墨烯—镍泡沫的复合材料,通过将NiCo2O4生长在三维多孔石墨烯上,增加材料的赝电容反应,提高比容量,并能防止电化学反应过程中石墨烯结构的坍塌,从而具有良好的循环稳定性。
综上所述,石墨烯基超级电容器相比于传统电极材料有着比容量大、能量密度高等诸多优势,但也存在电化学反应过程中石墨烯的团聚、能量衰减等问题需要解决。因此,在设计和合成出新颖的石墨烯基电极材料从而获得高性能超级电容器方面仍有较大空间。
2.3 石墨烯在新型储能器件中的应用 2.3.1 在锂硫电池中的应用近几年石墨烯在锂硫电池电极材料中的应用也有很多报道[93-95]。锂硫电池虽具有较高的能量密度(2 600 Wh ·kg-1),耐过充能力强等特点,但是存在硫的导电性差,反应过程中多硫离子容易溶解在电解液中等问题。有研究者通过向锂硫电池电极材料中加入石墨烯,尝试解决上述问题[96-97]。Zhao等[97]制备出了还原氧化石墨烯包覆硫的复合材料,极大地提高了材料的导电性,并能减小多硫离子的溶解,使锂硫电池的循环稳定性有了一定程度的提高。Wang等[98]将氧化石墨烯自组装成膜结构,用于硫和多硫化物的吸收层,可有效抑制多硫化物的穿梭效应,100次循环后容量为895 mAh ·g-1,容量保持率为71%。
当前,将石墨烯用于锂硫电池电极材料的研究已取得一定成果,但是要真正实现锂硫电池的商业化,还需要进一步深入研究。
2.3.2 在钠离子电池中的应用钠离子电池具有和锂离子电池相似的反应机理,且钠资源储量丰富,开采简单,在新一代储能电池中具有很好的应用前景[99-100]。钠离子电池的负极材料主要有:碳材料、金属及其氧化物、磷及磷化物和钛基材料等。其中碳材料因具有来源广、导电性好等优点,将其应用于钠离子电池负极材料引起了人们的关注。然而,为了解决碳材料钠存储容量较低的问题,有研究者提出将碳材料与石墨烯复合,以实现缩短钠离子的传输距离,增强材料的导电性,保持电极结构的稳定性的目的[101-102]。此外,将石墨烯与金属氧化物复合用于钠离子电池负极材料也有很多报道。
Wang等[103]制备出的二氧化锡与还原氧化石墨烯的复合电极,拥有多孔的缓冲结构,能促进Na+的快速扩散,并能承受较大的体积变化。经测试,电池具有良好的倍率性能,在高倍率下,容量可逆性较好。David等[104]将石墨烯与MoS2纳米片复合,常温下合成了多层复合纸结构,将它直接用于钠离子电池负极,经电化学测试发现,钠离子电池表现出较好的循环稳定性,并有望为可充电电池的大面积柔性电极的应用开辟新途径。
除以上应用外,石墨烯在锂空气电池[105-106]、太阳能电池[107-109]和燃料电池[110-111]等储能领域中也有极大的应用潜力。
3 结语石墨烯自发现以来,因其良好的电学性能,在锂离子电池、超级电容器等储能器件中具有其他材料不可比拟的优势,受到国内外科研人员的广泛关注。然而,如何能低成本、大规模地生产高质量石墨烯,是科学界亟需解决的问题,成为近几年研究的热点之一。电化学制备石墨烯的方法具有简单、经济、环境友好,能得到较高质量的石墨烯等优点,为石墨烯的规模化绿色生产提供可能。然而,目前电化学制备石墨烯的方法还缺少完备的理论基础,制备过程中电解质离子的插层和剥离机理等方面还需要进一步研究,需要从理论和实际2方面解决石墨烯制备和应用的难题。
| [1] |
Bianco A, Cheng H M, Enoki T, et al. All in the graphene family:A recommended nomenclature for two-dimensional carbon materials[J]. Carbon, 2013, 65: 1-6. DOI:10.1016/j.carbon.2013.08.038 |
| [2] |
Novoselov K S, Jiang D, Schedin F, et al. Two-Dimensional atomic crystals[J]. Proceedings of the National Academy of Sciences, 2005, 102(30): 10451-10453. DOI:10.1073/pnas.0502848102 |
| [3] |
Novoselov K S. Electric field effect in atomically thin carbon films[J]. Science, 2004, 306(5696): 666-669. DOI:10.1126/science.1102896 |
| [4] |
Lee J, Tao L, Hao Y, et al. Embedded-Gate graphene transistors for high-mobility detachable flexible nanoelectronics[J]. Applied Physics Letters, 2012, 100(15): 152104-152107. DOI:10.1063/1.3702570 |
| [5] |
Liu W, Song N, Wu Y, et al. Preparation of layer-aligned graphene composite film with enhanced thermal conductivity[J]. Vacuum, 2017, 138: 39-47. DOI:10.1016/j.vacuum.2017.01.023 |
| [6] |
Papageorgiou D G, Kinloch I A, Young R J. Mechanical properties of graphene and graphene-based nanocomposites[J]. Progress in Materials Science, 2017, 90: 75-127. DOI:10.1016/j.pmatsci.2017.07.004 |
| [7] |
Gandhi M R, Vasudevan S, Shibayama A, et al. Graphene and graphene-based composites:A rising star in water purification-A comprehensive overview[J]. ChemistrySelect, 2016, 1(15): 4358-4385. DOI:10.1002/slct.201600693 |
| [8] |
Gao Y. Graphene and polymer composites for supercapacitor applications:A review[J]. Nanoscale Research Letters, 2017, 12(1): 1-17. DOI:10.1186/s11671-016-1773-2 |
| [9] |
Dongfang Y, Bock C. Laser reduced graphene for supercapacitor applications[J]. Journal of Power Sources, 2017, 337: 73-81. DOI:10.1016/j.jpowsour.2016.10.108 |
| [10] |
Bonaccorso F, Colombo L, Yu G, et al. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage[J]. Science, 2015, 347. DOI:10.1126/science.1246501 |
| [11] |
Pandit B, Jo C H, Joo K S, et al. Changes in physical properties of graphene oxide with thermal reduction[J]. Journal of the Korean Physical Society, 2017, 71(3): 156-160. DOI:10.3938/jkps.71.156 |
| [12] |
Cheng C, Deng J, Lei B, et al. Toward 3D graphene oxide gels based adsorbents for high-efficient water treatment via the promotion of biopolymers[J]. Journal of Hazardous Materials, 2013, 263: 467-478. DOI:10.1016/j.jhazmat.2013.09.065 |
| [13] |
Chun H, Lee J Y, Lee J H, et al. Enhanced photocatalysis of graphene and TiO2 dual-coupled carbon nanofibers post-treated at various temperatures[J]. Industrial & Engineering Chemistry Research, 2016, 55(1): 45-53. |
| [14] |
Zhou Y, Xiong W, Park J, et al. Laser-Assisted nanofabrication of carbon nanostructures[J]. Journal of Laser Applications, 2012, 24(4): 042007. DOI:10.2351/1.4716046 |
| [15] |
Meyer J C, Geim A K, Katsnelson M I, et al. On the roughness of single-and bi-layer graphene membranes[J]. Solid State Communications, 2007, 143(1/2): 101-109. |
| [16] |
Ghalkhani M, Shahrokhian S. Adsorptive stripping differential pulse voltammetric determination of mebendazole at a graphene nanosheets and carbon nanospheres/chitosan modified glassy carbon electrode[J]. Sensors and Actuators B:Chemical, 2013, 185: 669-674. DOI:10.1016/j.snb.2013.05.054 |
| [17] |
Tu R, Liang Y, Zhang C, et al. Fast synthesis of high-quality large-area graphene by laser CVD[J]. Applied Surface Science, 2018, 445: 204-210. DOI:10.1016/j.apsusc.2018.03.184 |
| [18] |
Lavin-Lopez M P, Fernandez-Diaz M, Sanchez-Silva L, et al. Improving the growth of monolayer CVD-graphene over polycrystalline iron sheets[J]. New Journal of Chemistry, 2017, 41(12): 5066-5074. DOI:10.1039/C7NJ00281E |
| [19] |
Treier M, Pignedoli C A, Laino T, et al. Surface-Assisted cyclodehydrogenation provides a synthetic Route towards easily processable and chemically tailored nanographenes[J]. Nature Chemistry, 2011, 3(1): 61-67. DOI:10.1038/nchem.891 |
| [20] |
Deokar G, Avila J, Razado-Colambo I, et al. Towards high quality CVD graphene growth and transfer[J]. Carbon, 2015, 89: 82-92. DOI:10.1016/j.carbon.2015.03.017 |
| [21] |
Arao Y, Mori F, Kubouchi M. Efficient solvent systems for improving production of few-layer graphene in liquid phase exfoliation[J]. Carbon, 2017, 118: 18-24. DOI:10.1016/j.carbon.2017.03.002 |
| [22] |
Narayan R, Lim J, Jeon T, et al. Perylene tetracarboxylate surfactant assisted liquid phase exfoliation of graphite into graphene nanosheets with facile re-dispersibility in aqueous/organic polar solvents[J]. Carbon, 2017, 119: 555-568. DOI:10.1016/j.carbon.2017.04.071 |
| [23] |
Chen J P, Shi W L, Fang D, et al. A binary solvent system for improved liquid phase exfoliation of pristine graphene materials[J]. Carbon, 2015, 94: 405-411. DOI:10.1016/j.carbon.2015.07.006 |
| [24] |
Alaferdov A V, Gholamipour-Shirazi A, Canesqui M A, et al. Size-Controlled synthesis of graphite nanoflakes and multi-layer graphene by liquid phase exfoliation of natural graphite[J]. Carbon, 2014, 69: 525-535. DOI:10.1016/j.carbon.2013.12.062 |
| [25] |
Park J S, Yu L, Lee C S, et al. Liquid-Phase exfoliation of expanded graphites into graphene nanoplatelets using amphiphilic organic molecules[J]. Journal of Colloid and Interface Science, 2014, 417: 379-384. DOI:10.1016/j.jcis.2013.11.066 |
| [26] |
Yazdi G, Iakimov T, Yakimova R. Epitaxial graphene on SiC:A review of growth and characterization[J]. Crystals, 2016, 6(5): 53. DOI:10.3390/cryst6050053 |
| [27] |
Li D, Müller M B, Gilje S, et al. Processable aqueous dispersions of graphene nanosheets[J]. Nature Nanotechnology, 2008, 3(2): 101-105. |
| [28] |
Hajjar Z, Rashidi A M, Ghozatloo A. Enhanced thermal conductivities of graphene oxide nanofluids[J]. International Communications in Heat and Mass Transfer, 2014, 57: 128-131. DOI:10.1016/j.icheatmasstransfer.2014.07.018 |
| [29] |
Zhang Y, Guo L, Xia H, et al. Photoreduction of graphene oxides:methods, properties, and applications[J]. Advanced Optical Materials, 2014, 2(1): 10-28. DOI:10.1002/adom.201300317 |
| [30] |
de Silva K K H, Huang H, Joshi R K, et al. Chemical reduction of graphene oxide using Green reductants[J]. Carbon, 2017, 119: 190-199. DOI:10.1016/j.carbon.2017.04.025 |
| [31] |
Wang Q, Chen J, Niu Q, et al. The application of graphene oxidized combining with decellularized scaffold to repair of sciatic nerve injury in rats[J]. Saudi Pharmaceutical Journal, 2017, 25(4): 469-476. DOI:10.1016/j.jsps.2017.04.008 |
| [32] |
Talyzin A V, Mercier G, Klechikov A, et al. Brodie vs Hummers graphite oxides for preparation of multi-layered materials[J]. Carbon, 2017, 115: 430-440. DOI:10.1016/j.carbon.2016.12.097 |
| [33] |
Alam S N, Sharma N, Kumar L. Synthesis of graphene oxide (GO) by modified hummers method and its thermal reduction to obtain reduced graphene oxide (rGO)[J]. Graphene, 2017, 6(1): 1-18. |
| [34] |
Ghorbani M, Abdizadeh H, Golobostanfard M R. Reduction of graphene oxide via modified hydrothermal method[J]. Procedia Materials Science, 2015, 11: 326-330. DOI:10.1016/j.mspro.2015.11.104 |
| [35] |
Reina A, Jia X, Ho J, et al. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition[J]. Nano Letters, 2009, 9(1): 30-35. |
| [36] |
Bointon T H, Barnes M D, Russo S, et al. High quality monolayer graphene synthesized by resistive heating cold wall chemical vapor deposition[J]. Advanced Materials, 2015, 27(28): 4200-4206. DOI:10.1002/adma.201501600 |
| [37] |
Munuera J M, Paredes J I, Villar-Rodil S, et al. Electrolytic exfoliation of graphite in water with multifunctional electrolytes:en Route towards high quality, oxide-free graphene flakes[J]. Nanoscale, 2016, 8(5): 2982-2998. DOI:10.1039/C5NR06882G |
| [38] |
Parvez K, Wu Z, Li R, et al. Exfoliation of graphite into graphene in aqueous solutions of inorganic salts[J]. Journal of the American Chemical Society, 2014, 136(16): 6083-6091. DOI:10.1021/ja5017156 |
| [39] |
Pei S, Wei Q, Huang K, et al. Green synthesis of graphene oxide by seconds timescale water electrolytic oxidation[J]. Nature Communications, 2018, 9(1): 145. DOI:10.1038/s41467-017-02479-z |
| [40] |
Low C T J, Walsh F C, Chakrabarti M H, et al. Electrochemical approaches to the production of graphene flakes and their potential applications[J]. Carbon, 2013, 54: 1-21. DOI:10.1016/j.carbon.2012.11.030 |
| [41] |
Tian S, Yang S, Huang T, et al. One-Step fast electrochemical fabrication of water-dispersible graphene[J]. Carbon, 2017, 111: 617-621. DOI:10.1016/j.carbon.2016.10.044 |
| [42] |
Su C, Lu A, Xu Y, et al. High-Quality thin graphene films from fast electrochemical exfoliation[J]. ACS Nano, 2011, 5(3): 2332-2339. DOI:10.1021/nn200025p |
| [43] |
Hernandez Y, Nicolosi V, Lotya M, et al. High-Yield production of graphene by liquid-phase exfoliation of graphite[J]. Nature Nanotechnology, 2008, 3(9): 563-568. DOI:10.1038/nnano.2008.215 |
| [44] |
Li X, Zhang G, Bai X, et al. Highly conducting graphene sheets and Langmuir-Blodgett films[J]. Nature Nanotechnology, 2008, 3(9): 538-542. DOI:10.1038/nnano.2008.210 |
| [45] |
Cao J, He P, Mohammed M A, et al. Two-Step electrochemical intercalation and oxidation of graphite for the mass production of graphene oxide[J]. Journal of the American Chemical Society, 2017, 139(48): 17446-17456. DOI:10.1021/jacs.7b08515 |
| [46] |
Li D, Müller M B, Gilje S, et al. Processable aqueous dispersions of graphene nanosheets[J]. Nature Nanotechnology, 2008, 3(2): 101-105. |
| [47] |
Eda G, Fanchini G, Chhowalla M. Large-Area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material[J]. Nature Nanotechnology, 2008, 3(5): 270-274. DOI:10.1038/nnano.2008.83 |
| [48] |
Zhao J, Pei S, Ren W, et al. Efficient preparation of large-area graphene oxide sheets for transparent conductive films[J]. ACS Nano, 2010, 4(9): 5245-5252. DOI:10.1021/nn1015506 |
| [49] |
Dikin D A, Stankovich S, Zimney E J, et al. Preparation and characterization of graphene oxide paper[J]. Nature, 2007, 448(7152): 457-460. DOI:10.1038/nature06016 |
| [50] |
Achee T C, Sun W M, Hope J T, et al. High-Yield scalable graphene nanosheet production from compressed graphite using electrochemical exfoliation[J]. Scientific Reports, 2018, 8(1): 14525. DOI:10.1038/s41598-018-32741-3 |
| [51] |
Wang J, Manga K K, Bao Q, et al. High-Yield synthesis of few-layer graphene flakes through electrochemical expansion of graphite in propylene carbonate electrolyte[J]. Journal of the American Chemical Society, 2011, 133(23): 8888-8891. DOI:10.1021/ja203725d |
| [52] |
Allen M J, Tung V C, Kaner R B. Honeycomb carbon:A review of graphene[J]. Chemical Reviews, 2010, 110(1): 132-145. |
| [53] |
Wu W, Zhang C, Hou S. Electrochemical exfoliation of graphene and graphene-analogous 2D nanosheets[J]. Journal of Materials Science, 2017, 52(18): 10649-10660. DOI:10.1007/s10853-017-1289-x |
| [54] |
Bonanni A, Pumera M. Surfactants used for dispersion of graphenes exhibit strong influence on electrochemical impedance spectroscopic response[J]. Electrochemistry Communications, 2012, 16(1): 19-21. |
| [55] |
van Thanh D, Oanh P P, Huong D T, et al. Ultrasonic-Assisted cathodic electrochemical discharge for graphene synthesis[J]. Ultrasonics Sonochemistry, 2017, 34: 978-983. DOI:10.1016/j.ultsonch.2016.07.025 |
| [56] |
Toh S Y, Loh K S, Kamarudin S K, et al. The impact of electrochemical reduction potentials on the electrocatalytic activity of graphene oxide toward the oxygen reduction reaction in an alkaline medium[J]. Electrochimica Acta, 2016, 199: 194-203. DOI:10.1016/j.electacta.2016.03.103 |
| [57] |
Liu S, Wang J, Zeng J, et al. "Green" electrochemical synthesis of Pt/graphene sheet nanocomposite film and its electrocatalytic property[J]. Journal of Power Sources, 2010, 195(15): 4628-4633. DOI:10.1016/j.jpowsour.2010.02.024 |
| [58] |
Guo H, Wang X, Qian Q, et al. A Green approach to the synthesis of graphene nanosheets[J]. ACS Nano, 2009, 3(9): 2653-2659. DOI:10.1021/nn900227d |
| [59] |
Toh S Y, Loh K S, Kamarudin S K, et al. Graphene production via electrochemical reduction of graphene oxide:Synthesis and characterisation[J]. Chemical Engineering Journal, 2014, 251: 422-434. DOI:10.1016/j.cej.2014.04.004 |
| [60] |
Rychagov A Y, Gubin S P, Chuprov P N, et al. Electrochemical reduction and electric conductivity of graphene oxide films[J]. Russian Journal of Electrochemistry, 2017, 53(7): 721-727. DOI:10.1134/S1023193517070102 |
| [61] |
Ma Y, Han J, Wang M, et al. Electrophoretic deposition of graphene-based materials:A review of materials and their applications[J]. Journal of Materiomics, 2018, 4(2): 108-120. DOI:10.1016/j.jmat.2018.02.004 |
| [62] |
Sarkar P, Nicholson P S. Electrophoretic deposition (EPD):Mechanisms, kinetics, and application to ceramics[J]. Journal of the American Ceramic Society, 1996, 79(8): 1987-2002. DOI:10.1111/j.1151-2916.1996.tb08929.x |
| [63] |
Huang S, Wu G, Chen C, et al. Electrophoretic deposition and thermal annealing of a graphene oxide thin film on carbon fiber surfaces[J]. Carbon, 2013, 52: 613-616. DOI:10.1016/j.carbon.2012.09.062 |
| [64] |
An S, Zhu Y, Lee S H, et al. Thin film fabrication and simultaneous anodic reduction of deposited graphene oxide platelets by electrophoretic deposition[J]. The Journal of Physical Chemistry Letters, 2010, 1(8): 1259-1263. DOI:10.1021/jz100080c |
| [65] |
Wang M, Duong L, Mai N, et al. All-Solid-State reduced graphene oxide supercapacitor with large volumetric capacitance and ultralong stability prepared by electrophoretic deposition method[J]. ACS Applied Materials & Interfaces, 2015, 7(2): 1348-1354. |
| [66] |
Wang M, Duong L, Oh J, et al. Large-Area, conductive and flexible reduced graphene oxide (RGO) membrane fabricated by electrophoretic deposition (EPD)[J]. ACS Applied Materials & Interfaces, 2014, 6(3): 1747-1753. |
| [67] |
Yang Y, Huang J, Zeng J, et al. Direct electrophoretic deposition of binder-free Co3O4/Graphene sandwich-like hybrid electrode as remarkable lithium ion battery anode[J]. ACS Applied Materials & Interfaces, 2017, 9(38): 32801-32811. |
| [68] |
Li Z, Zhou Y, Yang Y, et al. Electrophoretic deposition of graphene-TiO2 hierarchical spheres onto Ti thread for flexible fiber-shaped dye-sensitized solar cells[J]. Materials & Design, 2016, 105: 352-358. |
| [69] |
Mehrali M, Akhiani A R, Talebian S, et al. Electrophoretic deposition of calcium silicate-reduced graphene oxide composites on titanium substrate[J]. Journal of the European Ceramic Society, 2016, 36(2): 319-332. DOI:10.1016/j.jeurceramsoc.2015.08.025 |
| [70] |
Wang Q, Vasilescu A, Wang Q, et al. Electrophoretic approach for the simultaneous deposition and functionalization of reduced graphene oxide nanosheets with diazonium compounds:application for lysozyme sensing in serum[J]. ACS Applied Materials & Interfaces, 2017, 9(14): 12823-12831. |
| [71] |
Liu X, Qian T, Xu N, et al. Preparation of on chip, flexible supercapacitor with high performance based on electrophoretic deposition of reduced graphene oxide/polypyrrole composites[J]. Carbon, 2015, 92: 348-353. DOI:10.1016/j.carbon.2015.05.039 |
| [72] |
Zhang Y, Wu C, Guo S, et al. Interactions of graphene and graphene oxide with proteins and peptides[J]. Nanotechnology Reviews, 2013, 2(1). DOI:10.1515/ntrev-2012-0078 |
| [73] |
Lian P, Zhu X, Liang S, et al. Large reversible capacity of high quality graphene sheets as an anode material for lithium-ion batteries[J]. Electrochimica Acta, 2010, 55(12): 3909-3914. DOI:10.1016/j.electacta.2010.02.025 |
| [74] |
Guo P, Song H, Chen X, et al. Effect of graphene nanosheet addition on the electrochemical performance of anode materials for lithium-ion batteries[J]. Analytica Chimica Acta, 2011, 688(2): 146-155. DOI:10.1016/j.aca.2010.12.033 |
| [75] |
Yang X, Qiu L, Cheng C, et al. Ordered gelation of chemically converted graphene for next-generation electroconductive hydrogel films[J]. Angewandte Chemie International Edition, 2011, 50(32): 7325-7328. DOI:10.1002/anie.201100723 |
| [76] |
Ma Y, Younesi R, Pan R, et al. Constraining Si particles within graphene foam monolith:Interfacial modification for high-performance Li+ storage and flexible integrated configuration[J]. Advanced Functional Materials, 2016, 26(37): 6797-6806. DOI:10.1002/adfm.201602324 |
| [77] |
Xia F, Kwon S, Lee W W, et al. Graphene as an interfacial layer for improving cycling performance of Si nanowires in lithium-ion batteries[J]. Nano Letters, 2015, 15(10): 6658-6664. DOI:10.1021/acs.nanolett.5b02482 |
| [78] |
Liu H, Shen Z, Liang S, et al. One-Step in situ preparation of liquid-exfoliated pristine graphene/Si composites:Towards practical anodes for commercial lithium-ion batteries[J]. New Journal of Chemistry, 2016, 40(8): 7053-7060. DOI:10.1039/C6NJ01234E |
| [79] |
Rahman M A, Song G, Bhatt A I, et al. Nanostructured silicon anodes for high-performance lithium-ion batteries[J]. Advanced Functional Materials, 2016, 26(5): 647-678. DOI:10.1002/adfm.201502959 |
| [80] |
Jiao L, Liu J, Li H, et al. Facile synthesis of reduced graphene oxide-porous silicon composite as superior anode material for lithium-ion battery anodes[J]. Journal of Power Sources, 2016, 315: 9-15. DOI:10.1016/j.jpowsour.2016.03.025 |
| [81] |
Liu X, Zhang J, Si W, et al. Sandwich nanoarchitecture of Si/Reduced graphene oxide bilayer nanomembranes for li-ion batteries with long cycle life[J]. ACS Nano, 2015, 9(2): 1198-1205. DOI:10.1021/nn5048052 |
| [82] |
Tian R, Wang W, Huang Y, et al. 3D composites of layered MoS2 and graphene nanoribbons for high performance lithium-ion battery anodes[J]. Journal of Materials Chemistry A, 2016, 4(34): 13148-13154. DOI:10.1039/C6TA04331C |
| [83] |
Grote F, Gruber C, Börrnert F, et al. Thermal disproportionation of oxo-functionalized graphene[J]. Angewandte Chemie International Edition, 2017, 56(31): 9222-9225. DOI:10.1002/anie.201704419 |
| [84] |
Suktha P, Phattharasupakun N, Dittanet P, et al. Charge storage mechanisms of electrospun Mn3O4 nanofibres for high-performance supercapacitors[J]. RSC Advances, 2017, 7(16): 9958-9963. DOI:10.1039/C6RA28499J |
| [85] |
Inagaki M, Kang F, Toyoda M, et al. Carbon materials for electrochemical capacitors[M]//Advanced Materials Science and Engineering of Carbon. Elsevier, 2014: 237-265. doi: 10.1016/b978-0-12-407789-8.00011-9
|
| [86] |
El-Kady M F, Strong V, Dubin S, et al. Laser scribing of high-performance and flexible graphene-based electrochemical capacitors[J]. Science, 2012, 335(6074): 1326-1330. DOI:10.1126/science.1216744 |
| [87] |
Zhu Y, Murali S, Stoller M D, et al. Carbon-Based supercapacitors produced by activation of graphene[J]. Science, 2011, 332(6037): 1537-1541. DOI:10.1126/science.1200770 |
| [88] |
Zhu C, Liu T, Qian F, et al. Supercapacitors based on three-dimensional hierarchical graphene aerogels with periodic macropores[J]. Nano Letters, 2016, 16(6): 3448-3456. DOI:10.1021/acs.nanolett.5b04965 |
| [89] |
Lehtimäki S, Suominen M, Damlin P, et al. Preparation of supercapacitors on flexible substrates with electrodeposited PEDOT/Graphene composites[J]. ACS Applied Materials & Interfaces, 2015, 7(40): 22137-22147. |
| [90] |
Wang X, Zhang Y, Zhi C, et al. Three-Dimensional strutted graphene grown by substrate-free sugar blowing for high-power-density supercapacitors[J]. Nature Communications, 2013, 4: 2905-2912. DOI:10.1038/ncomms3905 |
| [91] |
Qin K, Liu E, Li J, et al. Supercapacitors:Free-Standing 3D nanoporous duct-like and hierarchical nanoporous graphene films for micron-level flexible solid-state asymmetric supercapacitors[J]. Advanced Energy Materials, 2016, 6(18). DOI:10.1002/aenm.201670107 |
| [92] |
Yu M, Chen J, Liu J, et al. Mesoporous NiCo2O4 nanoneedles grown on 3D graphene-nickel foam for supercapacitor and methanol electro-oxidation[J]. Electrochimica Acta, 2015, 151: 99-108. DOI:10.1016/j.electacta.2014.10.156 |
| [93] |
Lei D, Shi K, Ye H, et al. Solid-State electrolytes:Progress and perspective of solid-state lithium-sulfur batteries[J]. Advanced Functional Materials, 2018, 28(38): 1870272. DOI:10.1002/adfm.201870272 |
| [94] |
Liu D, Zhang C, Zhou G, et al. Catalytic effects in lithium-sulfur batteries:Promoted sulfur transformation and reduced shuttle effect[J]. Advanced Science, 2018. |
| [95] |
Sun J, Sun Y, Pasta M, et al. Entrapment of polysulfides by a black-phosphorus-modified separator for lithium-sulfur batteries[J]. Advanced Materials, 2016, 28(44): 9797-9803. DOI:10.1002/adma.201602172 |
| [96] |
Xu C, Wu Y, Zhao X, et al. Sulfur/Three-Dimensional graphene composite for high performance lithium-sulfur batteries[J]. Journal of Power Sources, 2015, 275: 22-25. DOI:10.1016/j.jpowsour.2014.11.007 |
| [97] |
Zhao H, Peng Z, Wang W, et al. Reduced graphene oxide with ultrahigh conductivity as carbon coating layer for high performance sulfur@reduced graphene oxide cathode[J]. Journal of Power Sources, 2014, 245: 529-536. DOI:10.1016/j.jpowsour.2013.07.002 |
| [98] |
Wang X, Wang Z, Chen L. Reduced graphene oxide film as a shuttle-inhibiting interlayer in a lithium-sulfur battery[J]. Journal of Power Sources, 2013, 242: 65-69. DOI:10.1016/j.jpowsour.2013.05.063 |
| [99] |
Geim A K. Graphene:Status and prospects[J]. Science, 2009, 324(5934): 1530-1534. DOI:10.1126/science.1158877 |
| [100] |
Novoselov K S, Geim A K, Morozov S V, et al. Two-Dimensional gas of massless Dirac fermions in graphene[J]. Nature, 2005, 438(7065): 197-200. DOI:10.1038/nature04233 |
| [101] |
Xu J, Wang M, Wickramaratne N P, et al. High-Performance sodium ion batteries based on a 3D anode from nitrogen-doped graphene foams[J]. Advanced Materials, 2015, 27(12): 2042-2048. DOI:10.1002/adma.201405370 |
| [102] |
Kim Y, Ha K H, Oh S M, et al. High-Capacity anode materials for sodium-ion batteries[J]. Chemistry-a European Journal, 2014, 20(38): 11980-11992. DOI:10.1002/chem.201402511 |
| [103] |
Wang Y, Lim Y G, Park M S, et al. Ultrafine SnO2 nanoparticle loading onto reduced graphene oxide as anodes for sodium-ion batteries with superior rate and cycling performances[J]. J Mater Chem A, 2014, 2(2): 529-534. DOI:10.1039/C3TA13592F |
| [104] |
David L, Bhandavat R, Singh G. MoS2/Graphene composite paper for sodium-ion battery electrodes[J]. ACS Nano, 2014, 8(2): 1759-1770. DOI:10.1021/nn406156b |
| [105] |
Xin X, Ito K, Kubo Y. Graphene/Activated carbon composite material for oxygen electrodes in lithium-oxygen rechargeable batteries[J]. Carbon, 2016, 99: 167-173. DOI:10.1016/j.carbon.2015.12.015 |
| [106] |
Qiu D, Bu G, Zhao B, et al. In situ growth of mesoporous NiO nanoplates on a graphene matrix as cathode catalysts for rechargeable lithium-air batteries[J]. Materials Letters, 2015, 141: 43-46. DOI:10.1016/j.matlet.2014.11.033 |
| [107] |
Al-Rawashdeh N A F, Albiss B A, Yousef M H I. Graphene-Based transparent electrodes for dye sensitized solar cells[J]. IOP Conference Series:Materials Science and Engineering, 2018, 305. DOI:10.1088/1757-899X/305/1/012019 |
| [108] |
Lukaszkowicz K, Szindler M, Drygała A, et al. Graphene-Based layers deposited onto flexible substrates:Used in dye-sensitized solar cells as counter electrodes[J]. Applied Surface Science, 2017, 424: 157-163. DOI:10.1016/j.apsusc.2017.02.040 |
| [109] |
Liu Z, You P, Xie C, et al. Ultrathin and flexible perovskite solar cells with graphene transparent electrodes[J]. Nano Energy, 2016, 28: 151-157. DOI:10.1016/j.nanoen.2016.08.038 |
| [110] |
Pullamsetty A, Subbiah M, Sundara R. Platinum on boron doped graphene as cathode electrocatalyst for proton exchange membrane fuel cells[J]. International Journal of Hydrogen Energy, 2015, 40(32): 10251-10261. DOI:10.1016/j.ijhydene.2015.06.020 |
| [111] |
Marinoiu A, Raceanu M, Carcadea E, et al. Low cost iodine intercalated graphene for fuel cells electrodes[J]. Applied Surface Science, 2017. DOI:10.1016/j.apsusc.2017.01.295 |
 2019, Vol. 36
2019, Vol. 36