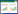蒸汽裂解制烯烃(乙烯或丙烯)过程中会产生微量的炔烃和共轭二烯烃。例如,乙烯中通常含有0.5%~3.0%的乙炔,丙烯中则含有2.0%~8.0%的丙炔和丙二烯。炔烃和共轭二烯烃较单烯烃更为活泼,易发生聚合,后续工段中加快了聚合催化剂失活。按聚乙烯、乙丙橡胶、聚丙烯等后加工工序的要求,聚合级乙烯中乙炔的体积分数必须低于5×10-6;乙二醇生产也要求乙烯中乙炔的体积分数低于10×10-6。乙烯中乙炔的脱除包括溶剂吸收、选择加氢、低温精馏、乙炔酮沉淀、氨化、络合吸收等方法。其中,选择加氢法工艺较简单、能耗低、污染小,为很多现代大型乙烯工厂所采用。催化剂结构性质是实现炔烃高效选择加氢、防止单烯烃进一步加氢的关键,国内外大量学者开展了相关催化剂的研究。本论文主要对乙炔选择加氢催化剂的研究进展进行了综述,旨在为性能优良催化剂的开发提供有价值信息。
1 乙炔加氢反应机理催化剂乙炔选择加氢性能与乙炔、乙烯在催化剂表面吸附方式及强度密切相关。Horiuti等[1]认为,乙炔加氢反应遵循Horiuti-Polanyi机理,即氢分子在催化剂表面吸附解离生成氢原子,然后与吸附的乙炔反应形成一系列中间体及产物(包括乙烯基、乙烯、乙烷基及乙烷等)。依据一些表面研究技术(如FT-IR、EELS、SFG、HREELS及ARUPS等)与动力学的研究结果,学者们推测在乙炔加氢过程中可能存在下列吸附物种[2]:
 |
通常认为,在金属表面乙炔通过π键吸附形成(1) 物种,该物种可转变为通过di-σ键吸附的乙炔物种(2),然后进一步形成吸附态乙烯基(3)。乙烯基(3) 既可以加氢生成乙烯,也可以加氢生成亚乙基(4),而亚乙基经脱氢可生成次乙基(5)。通过端基碳原子形成的多位吸附物种(4) 和(5) 易深度加氢转化为乙烷,也会发生C—C键断裂形成积碳及加氢形成甲烷等。此外,乙烯基(3) 还可发生自身聚合生成绿油的前体1, 3-丁二烯。解离吸附的乙炔物种(6) 和亚乙烯基(7) 也被认为是绿油及苯的前驱物。此外,乙烯在催化剂表面的主要吸附方式类似于乙炔。
为提高乙炔加氢反应中乙烯选择性,理想的乙炔吸附方式是形成π键络合物,然后依次经di-σ键吸附和乙烯基转变为乙烯。然而,乙炔在金属(如Pd)表面会形成吸附更稳定的次乙基。为抑制次乙基形成,应在催化剂表面构建“孤立”的单原子活中心而利于乙炔以π键或di-σ键方式吸附[3-4]。可见,影响乙炔和乙烯在其表面吸附模式及吸附强度的关键因素是催化剂的几何和电子结构。
2 乙炔加氢催化剂 2.1 主催化剂(活性组分)的研究选择加氢催化剂主要为Ⅷ族及IB族金属,其中Pd基催化剂性能较佳,已在工业上应用。然而,Pd基催化剂成本高,研究开发非贵金属乙炔选择加氢催化剂也越来越受到关注。
2.1.1 Pd基催化剂早在20世纪50年代,Bond等[5]发现α-Al2O3负载Pd的乙炔选择加氢活性和选择性优于Pt及Rh。随后,其他学者发现SiO2、TiO2及碳纳米管等负载Pd催化剂同样具有良好的炔烃选择加氢性能[6-8]。但是,单金属Pd催化剂在应用过程中存在如下缺点:1) 乙炔在Pd表面易形成强吸附的次乙基,导致乙炔发生深度加氢;并且,乙炔在Pd表面较易发生聚合反应而导致催化剂失活,随着反应进行乙烯选择性降低[9];2) 在液相乙炔加氢时,炔键与Pd原子强烈的络合作用导致Pd组分逐渐流失到反应介质中,催化剂发生永久失活;3) Pd易与H2作用形成氢化物,次表面活性氢会促使乙烯进一步加氢。
针对单金属Pd催化剂的缺点,研究者们采用Ga、Ag、Au、Cu、Zn及Cr等金属对Pd催化剂的几何和电子结构进行调变,以改善其乙炔选择加氢性能。
研究表明[3-4, 10-11],Ag明显提高Pd/Al2O3催化剂乙烯选择性及催化剂稳定性源于形成了PdAg合金。一方面,Ag “稀释”了金属Pd表面活性中心形成了较多“孤立”的Pd原子、利于乙炔及乙烯以π-络合物吸附;同时,Ag→Pd的电子转移使Pd呈富电子态,减弱了Pd对其表面中间物种的吸附强度、抑制了乙烷形成。Osswald等[12-13]研究发现,金属间化合物GaPd/Al2O3乙炔选择加氢活性与Pd/Al2O3相近,但乙烯选择性和稳定性明显较佳。Armbrüster等[14]认为PdGa金属间化合物高选择性及稳定性源于Ga的“活性位隔离”作用[15-17]及Ga与Pd间电子作用,前者增加了“孤立”Pd原子的数量,而后者调变了Pd原子费米能级周围的电子云密度。
如上所述,工业用催化剂多数以Pd为主活性组分(高活化氢能力)、惰性金属(低活化氢能力、较高单烯选择性)为助剂。近年来,一些学者以Cu为选择加氢催化剂主组分、将气相Pd作为助剂沉积在Cu表面,并借助表面科学技术证实了Pd被包裹在Cu表面层内。该种方法利用Pd的高解离氢能力,通过氢溢流将活化的氢转移到高乙烯选择性、低氢活化能力的Cu上,不仅降低了反应所需的温度,同时也大大提高了乙烯选择性及催化剂的稳定性[18-19]。McCue等[20-21]将不同含量的Pd添加到Cu/Al2O3制备了双金属Cu-Pd/Al2O3催化剂并考察其乙炔选择加氢性能。发现在n(Cu):n(Pd)=50:1,n(H2)/n(C2H2)=10:1,90 ℃时,乙炔转化率接近100%,乙烯选择性大于80%,与单金属Cu相比,不但反应温度大大降低,而且催化剂性能也更佳。
除了PdGa、PdAg及PdCu外,PdCr [22]、及PdZn[23-24]等双金属催化剂的乙烯选择性及稳定性均优于单金属。总之,Pd基双金属催化剂乙炔选择加氢性能的改进与主要源于第2种金属的几何及电子作用。
为了进一步提高催化剂的性能,研究者还开展了三元及以上Pd基金属催化剂研究。Lee等[25]等发现,即使在氢含量较低的条件下,Ni改性Pd-Ag/Al2O3催化剂仍具有很高的乙炔转化率,原因在于Ni具有较高的活化氢的能力,弥补了由于Ag稀释导致表面Pd活性位减少、催化剂活性降低的问题[26]。
2.1.2 Au催化剂传统方法制备的Au颗粒较大,反应物分子在其表面难以吸附、活化,故过去Au通常被认为无催化活性。在20世纪90年代,Stobinski [27]及Bus等[28-29]研究发现,随Au晶粒减小,棱边和顶角处低配位数的Au原子增多,对H2吸附解离能力增强。
Jia [30]及Gluhoia等[31]等研究表明,反应温度在40~250 ℃范围内,Au/A12O3催化剂上乙炔选择加氢产物乙烯的选择性均保持在100%;只有当温度高于300 ℃时乙烯选择性才略有降低。他们还发现,Au颗粒尺寸在3 nm左右时催化剂活性最高,Au颗粒越大催化剂活性越差。Azizi等[32]考察了Au/CeO2催化剂(Au颗粒尺寸2 nm左右)的乙炔选择加氢性能,发现在300 ℃及H2/C2H2物质的量之比为30的条件下乙炔转化率和选择性都达到100%,并且反应温度低于300 ℃时具有较好稳定性。Au表面炔烃吸附强度远大于单烯烃是其具有高乙烯选择性的原因[33-34]。然而,单金属Au催化剂的加氢活性较低。
采用Au基合金催化剂可获得较好的选择加氢性能。Nikolaev等[35-36]研究表明,在84℃、n(C2H2)/n(C2H4)=1/20条件下,NiO/Au催化剂上乙炔转化率和乙烯选择性分别可达90%及100%,且在较大温度范围其乙烯的选择性均能保持在100%,其活性和稳定性明显优于NiO及Au等催化剂,他们认为原因如下:1) Au0向NiO电子转移形成了Auδ+位,作为Lewis碱的乙炔在Auδ+上吸附强度大于Au0,从而更容易发生活化;2) NiO使Au金属簇偏离了原先的球型结构而拥有了更多低配位数的角顶和棱边,更有利于H2的解离及对乙炔的优先吸附。Liu及Pei等[37-38]也发现Au与其他金属(如Ag、Pd)形成合金后催化性能明显优于单金属Au催化剂。
2.1.3 非贵金属催化剂Studt等[39]用同一金属表面上乙炔和乙烯的吸附热与甲基(CH3—)吸附热各成一定比例关系,以甲基吸附热为基础,分析了70多种金属合金及金属间化合物的乙炔选择加氢反应性能,认为Co-Ga、Fe-Zn和Ni-Zn非贵金属选择加氢催化剂具有应用前景,其中Ni-Zn合金综合性能最佳。早在20世纪90年代,Rodríguez等[40]已发现NiZn合金具有良好的乙炔选择加氢性能。Spanjers等[41]研究发现,随Zn含量增加,Ni-Zn合金催化剂的乙烯选择性提高。基于密度泛函理论计算和元素追踪法的结果,他们认为Zn稀释了表面Ni活性位,减弱了催化剂表面对乙炔的吸附,降低了乙炔覆盖度、抑制了绿油的形成及深度加氢,从而提高了乙烯选择性。Xu等[42]在Ni-Zn中掺杂少量的Au并于500 ℃ H2氛围中还原,得到了Au-Ni-Zn三元合金催化剂。与Ni-Zn相比,该三元合金催化剂的乙炔加氢活性及乙烯选择性分别提高了3和4倍,同时,催化剂表面的积炭量也大大减少。
采用类工业乙烯原料气(乙炔体积分数0.5%),Armbrüster等[43]发现单晶金属间化合物Al13Fe4上乙炔转化率和乙烯选择性于很长时间内稳定在81%~84%。作者认为这与Al13Fe4结构和电子性质相关,Fe活性位完全被Al隔离,并且Al与Fe之间形成的共价键改变了Fe的电子结构。
CeO2具有独特的结构及氧化还原性能[44-45],通常作为催化剂载体或者助剂[46-47]。Vilé等[48]首次考察了CeO2的丙炔(乙炔)选择加氢性能,发现在常压、250 ℃条件下,丙炔(乙炔)转化率为96%(86%)时丙烯(乙烯)选择性可达91%(81%)。他们还考察了制备条件对CeO2催化炔烃加氢性能的影响。随焙烧温度提高,催化剂选择加氢活性和烯烃选择性降低,因为焙烧温度提高,CeO2比表面积减小;随CeO2表面被还原度提高(即氧空位数量增加),其选择加氢性能降低,DFT计算表明CeO2表面氧空位对炔烃选择加氢不利。同时,他们还发现TiO2、ZnO、V2O5等具有氧化-还原性能氧化物几乎都没有炔烃选择加氢活性。可见,CeO2具有独特的催化性能。
非晶态合金由于其独特的几何和电子性质在加氢反应中具有良好的催化性能。研究者们发现,Ni-P和Ni-B非晶态合金中B或者P可以增加表面活性Ni位数量及配位不饱和程度,并且B可以提高Ni的电子云密度[49]。胡长员等[50]发现非晶态合金NiB/CNTs的活性及乙烯选择性高于晶态催化剂Ni/CNTs,并认为与硼向镍部分电子转移形成富电子的镍活性中心有关,富电子的镍活性中心利于H2的解离吸附而减弱了对C2H2的吸附,从而抑制了聚合及深度加氢。
除了上述非贵金属催化剂外,一些氮化物[51]、磷化物[52-53]、水滑石催化剂[54-55]及多金属合金催化剂[56]等都表现出较好的炔烃选择加氢性能。
2.2 载体载体主要对催化活性组分起机械承载、分散、提高催化剂的热稳定性及抗毒性等作用。炔烃加氢催化剂常用的载体有Al2O3、TiO2及SiO2,其他的还有碳纳米管或纳米纤维以及一些天然物质(如海泡石、硅藻土等)。
Sangkhum等[57]采用溶胶-凝胶法制备了纳米晶α-A12O3负载Pd催化剂,在100 ℃时乙炔转化率及乙烯选择性分别达到100%及80%。Komhom等[58]比较了α-A12O3、γ-A12O3和混合相A12O3负载金属Pd催化剂的乙炔选择加氢反应性能,发现过渡相(γ、θ等)-Al2O3和α-Al2O3混合相载体中α-A12O3的含量为64%时负载的Pd催化剂上乙炔转化率和乙烯的选择性最佳,他们认为原因在于混合相载体综合了α-Al2O3对乙烯的弱吸附和过渡相较大比表面积利于Pd分散的优势。Komeili等[59]也得到了类似的结果。
与A12O3、SiO2等载体相比,TiO2经较高温度还原后可与贵金属之间产生强相互作用(SMSI效应)而具有独特的性能。Ihm等[60-61]发现,在乙炔加氢反应中,Pd/TiO2催化剂的乙烯选择性明显高于Pd/γ-Al2O3。Panpranot等[8]发现Pd/TiO2(锐钛矿)的乙烯选择性远高于Pd/TiO2(金红石),并认为与锐钛矿TiO2更容易还原产生大量的Ti3+有关。Pd与TiO2强相互作用利于形成较多的“孤立”Pd原子,Ti3+的给电子性质提高了Pd的电子云密度,这均促进了催化剂乙炔选择加氢性能[62]。
Al2O3-TiO2复合氧化物可以弥补TiO2比表面积小和机械强度差的缺点,又保留了TiO2抗积碳、抗中毒以及与贵金属具有强相互作用的性质,并且两者相互作用还可以产生单独氧化物所不具备的独特物理化学性质,因而是一种优良的催化剂载体。韦以等[63-64]发现,在适当的条件下,Pd/Al2O3-TiO2上乙烯选择性大于85%,乙炔转化率达到90%以上,性能优于Pd/Al2O3催化剂。南军等[65]研究表明,将TiO2负载到Al2O3表面可以降低其表面酸性,有利于减少积碳、延长催化剂寿命。
侯宁等[66]以铝溶胶为无机黏合剂、蛋壳型SiO2纳米粉体为涂层、堇青石为基体然后采用微波法在涂覆后的堇青石基体上负载活性组分Pd制备了纳米涂层整体式加氢催化剂,其乙炔选择性加氢性能优于工业催化剂。同时,它兼备了蛋壳型纳米催化剂和整体式催化剂两者的优点,使用过程中易于装填、降低了反应器内床层压降,为实现纳米催化剂在工业上的应用提供了一种新的思路。
碳纳米管(CNTs)因具有一维孔道结构和独特的电子效应,作为金属催化剂载体近年来颇引人瞩目。Shao等[67]研究了CNTs负载纳米晶体Pd2Ga的乙炔选择加氢反应性能。碳纳米管上氧化的空穴和局域双键可以起到固定Pd2Ga纳米晶粒、抑制其烧结和流失的作用,并且Pd2Ga纳米晶粒表面具有大量低配位数的活性位,所以该催化剂具有较好的加氢活性和稳定性。Lu等[7, 68]指出,碳纳米管外的电子浓度高于管内,使附着于管外的活性组分对乙炔和乙烯吸附较弱,从而抑制了聚合反应、提高了单烯选择性。
2.3 碱性及氧化物助剂金属单质及氧化物等第三组分(助剂)可以通过改变主活性组分或者载体的物理化学结构及性质提高催化剂选择加氢性能。2.1节介绍了双(多)金属合金催化剂的性能,此处主要介绍碱性及氧化物等助剂对催化剂性能的影响。
采用碱性助剂可以抑制酸性载体(如Al2O3)或活性组分表面不饱和烃聚合等副反应。Park等[69]研究发现,在乙炔选择加氢反应中,经K、Ca及Mg等助剂改性后Pd/Al2O3催化剂上绿油生成量明显减少。李守振[70]以CaO对Pd-Ag/Al2O3乙炔选择加氢催化剂进行改性,发现CaO助剂不仅增加了Pd分散度,还有效抑制催化剂表面吸附态碳氢化合物向载体内部迁移。碳氢化合物从Pd晶粒向载体迁移时,会导致催化剂的孔体积和比表面积减小,阻碍反应的进行[71]。
具有氧化-还原性质的氧化物(如TiO2、CeO2和La2O3等)[72-74]作为助剂经高温还原可与Pd形成强烈相互作用,通过抑制Pd晶粒烧结及乙烯多键合吸附以提高单烯选择性。Kang等[72]发现,TiO2助剂可明显改善Pd/SiO2催化剂选择性加氢性能(尤其选择性),Ti物种对Pd原子的“隔离”作用大大抑制了绿油生成。Kim等[74]通过将La的氧化物和Si 2种助剂选择性沉积在Pd上对Pd/SiO2进行改性。经高温还原后La与Pd之间形成强烈的相互作用,而Si可以防止空气的氧化,保持这种强相互作用,使改性后的催化剂有更好的实用价值。
3 制备方法对催化剂性能的影响制备方法同样影响催化剂结构及乙炔选择加氢性能。
李朝晖等[75]比较了微乳液法和浸渍法制备Ni-Cu/Al2O3催化剂的乙炔选择加氢性能,发现前者制备的催化剂中活性组分分散度高,具有较好的催化活性和乙烯选择性。
Menezes等[76]比较了2种不同制备方法对负载PdCo催化剂性能影响。结果显示,与方法Ⅰ(将载体的醇溶液和活性组分前驱液混合,在载体表面直接合成纳米合金粒子)相比,采用方法Ⅱ(以PVP为稳定剂,先合成PdCo纳米粒子的胶体溶液,然后将其负载在载体上)制备的催化剂颗粒细小、分散均匀、稳定性好,在乙炔选择加氢反应中的活性、选择性及稳定性较佳。
近年来,采用射频等离子体技术制备和处理催化剂引起许多研究者的兴趣[77-81]。该方法特点在于浸渍法制备的催化剂前驱体经干燥后无需高温焙烧,而是直接对其进行等离子处理后直接用于催化反应。Chen等[77]考察了射频等离子体技术制备的Pd/Al2O3催化剂乙炔选择加氢性能,在50 ℃时乙炔转化率和乙烯选择性分别可达100%和71.3%,并长时间保持稳定,这可能与其表面存在高能粒子(离子、电子、自由基等)有关。Li等[79-81]研究表明,与传统的高温焙烧再还原方法相比,室温下用气体(H2、O2)等离子体技术处理Pd/TiO2催化剂前驱体在低温下便分解形成活性相、产生金属载-体强相互作用,提高活性组分分散度,并在活性相表面形成较多缺陷,具有较高的活性和乙烯选择性。
4 结语催化剂组成及结构是影响其乙炔选择加氢活性、乙烯选择性及稳定性的决定性因素。Pd基催化剂乙炔选择加氢性能最佳,并已在工业上应用。然而,贵金属催化剂成本较高,研究开发低成本催化剂(如Ni基)是发展趋势。值得注意的是,单金属(无论贵金属还是其他金属)催化剂乙炔选择加氢性能(尤其乙烯选择性)难以满足实际要求,通过形成二元或多元金属合金或金属间化合物以调节主活性组分的几何及电子结构是解决上述问题的主要手段之一。利用载体结构性质及活性组分与载体的相互作用调节乙炔及乙烯吸附方式及强度也是改善催化剂选择加氢性能的一种途径。再有,采用适宜制备方法控制活性相分散度、金属间以及金属与载体之间的相互作用同样可以改善催化剂选择加氢性能。总之,无论采用哪种方法,通过调节乙炔及乙烯在催化剂表面吸附方式和强度以改善催化剂选择加氢性能是研究方向。
此外,除了调变催化剂结构性质以改善其选择加氢性能外,原料中加入CO为竞争吸附剂以提高乙烯选择性已在工业上应用[82],而原料中加入给电子体调变金属活性中心电子性质也是一种策略[83-86]。
| [1] | Horiuti L, Polanyi M. Exchange reactions of hydrogen on metallic catalysts[J]. Transactions of the Faraday Society, 1934, 30: 1164–1172. DOI: 10.1039/tf9343001164 |
| [2] | Árpád M, Antal S, Mónika V. Hydrogenation of carbon-carbon multiple bonds: chemo-, regio-and stereo-selectivity[J]. Journal of Molecular Catalysis A: Chemical, 2001, 173(1/2): 185–221. |
| [3] | Mei D H, Neurock M, Smith C M. Hydrogenation of acetylene-ethylene mixtures over Pd and Pd-Ag alloys: First-principles-based kinetic Monte Carlo simulations[J]. Journal of Catalysis, 2009, 268(2): 181–195. DOI: 10.1016/j.jcat.2009.09.004 |
| [4] | Pei G, Liu X, Wang A, et al. Ag alloyed Pd single-atom catalysts for efficient selective hydrogenation of acetylene to ethylene in excess ethylene[J]. ACS Catalysis, 2015, 5(6): 3717–3725. DOI: 10.1021/acscatal.5b00700 |
| [5] | Bond G C, Dowden D A, Maekenzie N. The selective hydrogenation of acetylene[J]. Transaction of the Faraday Soeiety, 1958, 54: 1537–1546. DOI: 10.1039/tf9585401537 |
| [6] | Sárkány A, Horváth A, Beck A. Hydrogenation of acetylene over low loaded Pd and Pd-Au/SiO2 catalysts[J]. Applied Catalysis A: General, 2002, 229(1/2): 117–125. |
| [7] | Lu H, Xu B, Wang X, et al. The influence of Pd particles distribution position on Pd/CNTs catalyst for acetylene selective hydrogenation[J]. Catalysis Letters, 2014, 144(12): 2198–2203. DOI: 10.1007/s10562-014-1388-0 |
| [8] | Panpranot J, Kontapakdee K, Praserthdam P. Selective hydrogenation of acetylene in excess ethylene on micron-sized and nanocrystalline TiO2 supported Pd catalysts[J]. Applied Catalysis A: General, 2006, 314(1): 128–133. DOI: 10.1016/j.apcata.2006.08.024 |
| [9] | Ahn I Y, Lee J H, Kim S K, et al. Three-stage deactivation of Pd/SiO2 and Pd-Ag/SiO2 catalysts during the selective hydrogenation of acetylene[J]. Applied Catalysis A: General, 2009, 360(1): 38–42. DOI: 10.1016/j.apcata.2009.02.044 |
| [10] | Johnson M M, Walker D W, Nowack G P. Selective hydrogenation catalyst: US, 4404124A[P]. 1983-09-13 |
| [11] | Kim W J, Moon S H. Modified Pd catalysts for the selective hydrogenation of acetylene[J]. Catalysis Today, 2012, 185(1): 2–16. DOI: 10.1016/j.cattod.2011.09.037 |
| [12] | Osswald J, Giedigkeit R, Jentoft R E, et al. Palladium-gallium intermetallic compounds for the selective hydrogenation of acetylene: Part Ⅰ: Preparation and structural investigation under reaction conditions[J]. Journal of Catalysis, 2008, 258(1): 210–218. DOI: 10.1016/j.jcat.2008.06.013 |
| [13] | Osswald J, Kovnir K, Armbruüster M, et al. Palladium-gallium intermetallic compounds for the selective hydrogenation of acetylene: Part Ⅱ: Surface characterization and catalytic performance[J]. Journal of Catalysis, 2008, 258(1): 219–227. DOI: 10.1016/j.jcat.2008.06.014 |
| [14] | Armbrüster M, Kovnir K, Behrens M, et al. Pd-Ga intermetallic compounds as highly selective semihydrogenation catalyst[J]. Journal of the American Chemical Society, 2010, 132(42): 14745–14747. DOI: 10.1021/ja106568t |
| [15] | Kovnir K, Armbruüster M, Teschner D, et al. A new approach to well-defined, stable and site-isolated catalysts[J]. Science and Technology of Advanced Materials, 2007, 8(5): 420–427. DOI: 10.1016/j.stam.2007.05.004 |
| [16] | Prinz J, Pignedoli C A, Stöckl Q S, et al. Adsorption of small hydrocarbons on the three-fold PdGa surfaces: The road to selective hydrogenation[J]. Journal of the American Chemical Society, 2014, 136(33): 11792–11798. DOI: 10.1021/ja505936b |
| [17] | Prinz J, Gaspari R, Stöckl Q S, et al. Ensemble effect evidenced by CO adsorption on the 3-fold PdGa surfaces[J]. Journal of Physical Chemistry C, 2014, 118(23): 12260–12265. DOI: 10.1021/jp501584f |
| [18] | Kyriakou G, Boucher M B, Jewell A D, et al. Isolated metal atom geomretries as a strategy for selective heterogeneous hydrogenations[J]. Science, 2012, 335(6073): 1209–1212. DOI: 10.1126/science.1215864 |
| [19] | Boucher M B, Zugic B, Cladaras G, et al. Single atom alloy surface analogs in Pd0.18Cu15 nanoparticles for selective hydrogenation reactions[J]. Physical Chemistry Chemical Physics, 2013, 15(29): 12187–12196. DOI: 10.1039/c3cp51538a |
| [20] | McCue A J, McRitchie C J, Shepherd A M, et al. Cu/Al2O3 catalysts modified with Pd for selective acetylene hydrogenation[J]. Journal of Catalysis, 2014, 319: 127–135. DOI: 10.1016/j.jcat.2014.08.016 |
| [21] | McCue A J, Shepherd A M, Anderson J A. Optimisation of preparation method for Pd doped Cu/Al2O3 catalysts for selective acetylene hydrogenation[J]. Catalysis, Science & Technology, 2015, 5(5): 2880–2890. |
| [22] | Borgna A, Moraweek B, Massardier J, et al. New supported palladium-chromium catalysts: characterization and catalytic properties[J]. Journal of Catalysis, 1991, 128(1): 99–112. DOI: 10.1016/0021-9517(91)90070-K |
| [23] | Tew M W, Emerich H, Bokhoven J A. Formation and characterization of PdZn alloy: A very selective catalyst for alkyne semihydrogenation[J]. Journal of Physical Chemistry C, 2011, 115(17): 8457–8465. DOI: 10.1021/jp1103164 |
| [24] | Mashkovsky I S, Baeva G N, Stakheev A Y, et al. Novel Pd-Zn/C catalyst for selective alkyne hydrogenation: evidence for the formation of Pd-Zn bimetallic alloy particles[J]. Mendeleev Communications, 2014, 24(6): 355–357. DOI: 10.1016/j.mencom.2014.11.015 |
| [25] | Lee J H, Kim S K, Ahn I Y, et al. Performance of Ni-added Pd-Ag/Al2O3 catalysts in the selective hydrogenation of acetylene[J]. Korean Journal of Chemical Engineering, 2012, 29(2): 169–172. DOI: 10.1007/s11814-011-0170-x |
| [26] | Valcarcel A, Morfin F, Piccolo L. Alkene hydrogenation on metal surfaces: Why and when are Pd overlayers more efficient catalysts than bulk Pd?[J]. Journal of Catalysis, 2009, 263(2): 315–320. DOI: 10.1016/j.jcat.2009.02.023 |
| [27] | Stobinski L, Zommer L, Dus R. Molecular hydrogen interactions with discontinuous and continuous thin gold films[J]. Applied Surface Science, 1999, 141(3/4): 319–325. |
| [28] | Bus E, Miller J T, van Bokhoven J A. Hydrogen chemisorption on Al2O3-supported gold catalysts[J]. Journal of Physical Chemistry B, 2005, 109(30): 14581–14587. DOI: 10.1021/jp051660z |
| [29] | Bus E, van Bokhoven J A. Hydrogen chemisorption on supported platinum, gold, and platinum-gold-alloy catalysts[J]. Physical Chemistry Chemical Physics, 2007, 9(22): 2894–2902. DOI: 10.1039/B701402C |
| [30] | Jia J, Haraki K, Kondo J H, et al. Selective hydrogenation of acetylene over Au/Al2O3 catalyst[J]. Journal of Physical Chemistry B, 2000, 104(47): 11153–11156. DOI: 10.1021/jp001213d |
| [31] | Gluhoia A C, Bakkerb J W, Nieuwenhuys B E. Gold, still a surprising catalyst: Selective hydrogenation of acetylene to ethylene over Au nanoparticles[J]. Catalysis Today, 2010, 154(1/2): 13–20. |
| [32] | Azizi Y, Petit C, Pitchon V. Formation of polymer-grade ethylene by selective hydrogenation of acetylene over Au/CeO2 catalyst[J]. Journal of Catalysis, 2008, 256(2): 338–344. DOI: 10.1016/j.jcat.2008.04.003 |
| [33] | Hashmi A S K, Hutchings G J. Gold Catalysis[J]. Angewandte Chemie International Edition, 2006, 45(47): 7896–7936. DOI: 10.1002/(ISSN)1521-3773 |
| [34] | Segura Y, López N, Pérez-Ramírez J. Origin of the superior hydrogenation selectivity of gold nanoparticles in alkyne+alkene mixtures: Triple-versus double-bond activation[J]. Journal of Catalysis, 2007, 247(2): 383–386. DOI: 10.1016/j.jcat.2007.02.019 |
| [35] | Nikolaev S A, Smirnov V V. Synergistic and size effects in selective hydrogenation of alkynes on gold nanocomposites[J]. Catalysis Today, 2009, 147(Supplement): 336–341. |
| [36] | Nikolaev S A, Pichugina D A, Mukhamedzyanova D F. Sites for the selective hydrogenation of ethyne to ethene on supported NiO/Au catalysts[J]. Gold Bulletin, 2012, 45(15): 221–231. |
| [37] | Liu X, Li Y, Lee J W, et al. Selective hydrogenation of acetylene in excess ethylene over SiO2 supported Au-Ag bimetallic catalyst[J]. Applied Catalysis A: General, 2012, 439/440: 8–14. DOI: 10.1016/j.apcata.2012.06.030 |
| [38] | Pei G, Liu X, Wang A, et al. Promotional effect of Pd single atoms on Au nanoparticles supported on silica for the selective hydrogenation of acetylene in excess ethylene[J]. New Journal of Chemistry, 2014, 38: 2043–2051. DOI: 10.1039/c3nj01136d |
| [39] | Studt F, Ablid-Pedersen F, Bligaard T, et al. Identification of non-precious metal alloy catalysts for selective hydrogenation of acetylene[J]. Science, 2008, 320(5881): 1320–1322. DOI: 10.1126/science.1156660 |
| [40] | Rodríguez J C, Marchi A J, Borgna A, et al. Effect of Zn content on catalytic activity and physicochemical properties of Ni-based catalysts for selective hydrogenation of acetylene[J]. Journal of Catalysis, 1997, 171(1): 268–278. DOI: 10.1006/jcat.1997.1815 |
| [41] | Spanjers C S, Held J T, Jones M J, et al. Zinc inclusion to heterogeneous nickel catalysts reduces oligomerization during the semi-hydrogenation of acetylene[J]. Journal of Catalysis, 2014, 316: 164–173. DOI: 10.1016/j.jcat.2014.05.007 |
| [42] | Xu J, Huang Y, Yang X, et al. Enhancement of acetylene hydrogenation activity over Ni-Zn bimetallic catalyst by doping with Au[J]. Journal of Nanoscience & Nanotechnology, 2014, 14(9): 6894–6899. |
| [43] | Armbrüster M, Kovnir K, Friedrich M, et al. Al13Fe4 as a low-cost alternative for palladium in heterogeneous hydrogenation[J]. Nature Materials, 2012, 11: 690–693. DOI: 10.1038/nmat3347 |
| [44] | Farmer J A, Campbell C T. Ceria maintains smaller metal catalyst particles by strong metal-support bonding[J]. Science, 2010, 329: 933–936. DOI: 10.1126/science.1191778 |
| [45] | Ganduglia-Pirovano M V, Hofmann A, Sauer J. Oxygen vacancies in transition metal and rare earth oxides: Current state of understanding and remaining challenges[J]. Surface Science Reports, 2007, 62: 219–270. DOI: 10.1016/j.surfrep.2007.03.002 |
| [46] | Fu Q, Saltsburg H, Flytzani-Stephanopoulos M. Active nonmetallic Au and Pt species on ceria-based water-gas shift catalysts[J]. Science, 2003, 301: 935–938. DOI: 10.1126/science.1085721 |
| [47] | Yang F, Graciani J, Evans J, et al. CO oxidation on inverse CeOx/Cu(111) catalysts: High catalytic activity and ceria-promoted dissociation of O2[J]. Journal of the American Chemical Society, 2011, 133(10): 3444–3451. DOI: 10.1021/ja1087979 |
| [48] | Vilé G, Bridier B, Wichert J, et al. Ceria in hydrogenation catalysis: High selectivity in the conversion of alkynes to olefins[J]. Angewandte Chemmie International Edition, 2012, 51(34): 8620–8623. DOI: 10.1002/anie.201203675 |
| [49] | Li H, Li H, Dai W, et al. XPS studies on surface electronic characteristics of Ni-B and Ni-P amorphous alloy and its correlation to their catalytic properties[J]. Applied Surface Science, 1999, 152(1/2): 25–34. |
| [50] |
胡长员, 段武茂, 李凤仪, 等. 碳纳米管负载镍基催化剂上乙炔选择性加氢[J].
现代化工, 2007, 8: 34–37.
Hu Changyuan, Duan Wumao, Li Fengyi, et al. Acetylene selective hydrogenation on carbon nanotubes supported Ni-based catalyst[J]. Modern Chemical Industry, 2007, 8: 34–37. DOI: 10.3321/j.issn:0253-4320.2007.03.008 |
| [51] |
郝志显, 魏昭彬, 王来军, 等. γ-Mo2N催化剂上的乙炔选择加氢[J].
催化学报, 2011, 21(03): 217–220.
Hao Zhixian, Wei Zhaobin, Wang Laijun, et al. Selective hydrogenation of ethylene on γ-Mo2N[J]. Chinese Journal of Catalysis, 2011, 21(03): 217–220. |
| [52] |
周桂林, 王普光, 蒋宗轩, 等. MoP催化剂上乙炔选择性催化加氢[J].
催化学报, 2011, 32(1): 27–30.
Zhou Guilin, Wang Puguang, Jiang Zongxian, et al. Selective hydrogenation of acetylene over a MoP catalyst[J]. Chinese Journal of Catalysis, 2011, 32(1): 27–30. |
| [53] | Carenco S, Leyva-Pérez A, Concepción P, et al. Nickel phosphide nanocatalysts for the chemoselective hydrogenation of alkynes[J]. Nano Today, 2012, 7(1): 21–28. DOI: 10.1016/j.nantod.2011.12.003 |
| [54] | Abelló S, Verboekend D, Bridier B, et al. Activated takovite catalysts for partial hydrogenation of ethyne, propyne, and propadiene[J]. Journal of Catalysis, 2008, 259(1): 85–95. DOI: 10.1016/j.jcat.2008.07.012 |
| [55] | Bridier B, López N, Pérez-Ramírez J. Partial hydrogenation of propyne over copper-based catalysts and comparison with nickel-based analogues[J]. Journal of Catalysis, 2010, 269(1): 80–92. DOI: 10.1016/j.jcat.2009.10.019 |
| [56] | Bridier B, Pérez-Ramírez J. Cooperative effects in ternary Cu-Ni-Fe catalysts lead to enhanced alkene selectivity in alkyne hydrogenation[J]. Journal of the American Chemical Society, 2010, 132(12): 4321–4327. DOI: 10.1021/ja9101997 |
| [57] | Sangkhum T, Mekasuwandumrong O, Praserthdam P, et al. Effect of Fe-modified α-Al2O3 on the properties of Pd/α-Al2O3 catalysts in selective acetylene hydrogenation[J]. Reaction Kinetics and Catalysis Letters, 2009, 97(1): 115–123. DOI: 10.1007/s11144-009-0010-8 |
| [58] | Komhom S, Mekasuwandumrong O, Praserthdam P, et al. Improvement of Pd/A12O3 catalyst performance in selective acetylene hydrogenation using mixed phases A12O3 support[J]. Catalysis Communications, 2008, 10(1): 86–91. DOI: 10.1016/j.catcom.2008.07.039 |
| [59] | Komeili S, Ravanchi M T, Tae A. The influence of alumina phases on the performance of the Pd-Ag/Al2O3 catalyst in tail-end selective hydrogenation of acetylene[J]. Applied Catalysis A: General, 2015, 502: 287–296. DOI: 10.1016/j.apcata.2015.06.013 |
| [60] | Ihm S K, Lee D K. Process for manufacturing a titania supported palladium catalyst:US, 4839329[P]. 1989-07-13 |
| [61] |
顾虹, 许波连, 周静, 等. 负载型Pd/TiO2和Pd-Ag/TiO2催化剂的乙炔选择性加氢催化性能[J].
物理化学学报, 2006, 22(6): 712–715.
Gu Hong, Xu Bolian, Zhou Jing, et al. Catalytic properties of supported Pd/TiO2 and Pd-Ag/TiO2 catalysts for selective hydrogenation of acetylene[J]. Acta Phys -Chim Sin, 2006, 22(6): 712–715. |
| [62] | Riyapan S, Boonyongmaneerat Y, Mekasuwandumrong O, et al. Effect of surface Ti3+ on the sol-gel derived TiO2 in the selective acetylene hydrogenation on Pd/TiO2 catalysts[J]. Catalysis Today, 2015, 245: 134–138. DOI: 10.1016/j.cattod.2014.07.017 |
| [63] |
韦以, 刘新香. Al2O3-TiO2复合载体的制备与表征[J].
石油化工, 2006, 35(2): 173–177.
Wei Yi, Liu Xinxiang. Preparation and Characterization of Al2O3-TiO2 Complex Support[J]. Petrochemical Technology, 2006, 35(2): 173–177. |
| [64] |
韦以, 刘新香. Al2O3-TiO2复合载体用于乙炔选择加氢反应[J].
石油化工, 2006, 35(5): 411–415.
Wei Yi, Liu Xinxiang. Al2O3-TOi2 support for Pd catalyst used in acetylene selective hydrogenation[J]. Petrochemical Technology, 2006, 35(5): 411–415. |
| [65] |
南军, 谢海峰, 柴永明, 等. 一种TiO2修饰的Pd/A12O3选择性加氢用催化剂的研究[J].
催化学报, 2005, 26(8): 672–676.
Nan Jun, Xie Haifeng, Chai Yongming, et al. Study on TiO2-Modified Pd/Al2O3 catalyst for selective hydrogenation of pyrolysis gasoline[J]. Chinese Journal of Catalysis, 2005, 26(8): 672–676. |
| [66] | 侯宁, 朱秋锋, 付瑶, 等. 蛋壳型纳米催化剂结构化及其选择性加氢性能研究[C]. //沈志刚. 中国颗粒学会第七届学术年会暨海峡两岸颗粒技术研讨会论文集. 北京: 中国颗粒学会、中国科学院地球环境研究所、西安建筑科技大学, 2010: 626-629 |
| [67] | Shao L, Zhang W, Armbrüster M, et al. Nanosizing intermetallic compounds onto carbon nanotubes: Active and selective hydrogenation catalysts[J]. Angewandte Chemie International Edition, 2011, 50(43): 10231–10235. DOI: 10.1002/anie.201008013 |
| [68] |
胡长员, 李凤仪, 张荣斌, 等. 碳纳米管对非晶态NiB合金催化剂性能的影响[J].
分子催化, 2005, 19(5): 346–350.
Hu Changyuan, Li Fengyi, Zhang Rongbin, et al. The effects of CNTs on performance of amorphous NiB catalyst[J]. Journal of Molecular Catalysis, 2005, 19(5): 346–350. |
| [69] | Park Y H, Price G L. Potassium promoter for palladium on alumina selective hydrogenation catalysis[J]. Journal of the Chemical Society, Chemical Comunications, 1991, 17: 1188–1189. |
| [70] |
李守振, 戴伟, 张齐, 等. CaO改性的乙炔选择加氢催化剂Pd-Ag/Al2O3[J].
石油化工, 2012, 41(3): 272–276.
Li Shouzhen, Dai Wei, Zhang Qi, et al. CaO-Added Pd-Ag/Al2O3 catalyst for selective hydrogenation of acetylene[J]. Petrochemical Technology, 2012, 41(3): 272–276. |
| [71] | Kim W J, Kang J, Ahn I Y, et al. Deactivation behavior of a TiO2-added Pd catalyst in acetylene hydrogenation[J]. Journal of Catalysis, 2004, 226(1): 226–229. DOI: 10.1016/j.jcat.2004.05.017 |
| [72] | Kang J, Shin E W, Kim W J, et al. Selective hydrogenation of acetylene on TiO2-added Pd catalysts[J]. Journal of Catalysis, 2002, 208(2): 310–320. DOI: 10.1006/jcat.2002.3583 |
| [73] | Ahn I Y, Kim W J, Moon S H. Performance of La2O3-or Nb2O5-added Pd/SiO2 catalysts in acetylene hydrogenation[J]. Applied Catalysis A: General, 2006, 308(10): 75–81. |
| [74] | Kim W J, Ahn I Y, Lee J H, et al. Properties of Pd/SiO2 catalyst doubly promoted with La oxide and Si for acetylene hydrogenation[J]. Catalysis Communications, 2012, 24(5): 52–55. |
| [75] |
李朝晖, 戴伟, 傅吉全, 等. 微乳液法制备纳米Ni-Cu/Al2O3碳二选择加氢催化剂及其性能[J].
石油化工, 2009, 38(7): 723–727.
Li Chaohui, Dai Wei, Fu Jiquan, et al. Preparation of nano Ni-Cu/A12O3 catalyst for selective hydrogenation of C2 by microemulsion[J]. Petrochemical Technology, 2009, 38(7): 723–727. |
| [76] | Menezes W G, Altmann L, Zielasek V, et al. Bimetallic Co-Pd catalysts: Study of preparation methods and their influence on the selective hydrogenation of acetylene[J]. Journal of Catalysis, 2013, 300: 125–135. DOI: 10.1016/j.jcat.2012.12.023 |
| [77] | Chen M, Chu W, Dai X, et al. New palladium catalysts prepared by glow discharge plasma for the selective hydrogenation of acetylene[J]. Catalysis Today, 2004, 89(1/2): 201–204. |
| [78] | Shi C, Jang B W L. Nonthermal RF plasma modifications on Pd/γ-Al2O3 for selective hydrogenation of acetylene in the presence of ethylene[J]. Industrial Engineering Chemistry Research, 2006, 45(17): 5879–5884. DOI: 10.1021/ie0602512 |
| [79] | Li Y, Jang B W L. Non-thermal RF plasma effects on surface properties of Pd/TiO2 catalysts for selective hydrogenation of acetylene[J]. Applied Catalysis A: General, 2011, 392(1/2): 173–179. |
| [80] | Zhou T, Jang K, Jang B W L. Ionic liquid and plasma effects on SiO2 supported Pd for selective hydrogenation of acetylene[J]. Catalysis Today, 2013, 211(1): 147–155. |
| [81] | Zhu B, Jang B W L. Insights into surface properties of non-thermal RF plasmas treated Pd/TiO2in acetylene hydrogenation[J]. Journal of Molecular Catalysis A: Chemical, 2014, 395: 137–144. DOI: 10.1016/j.molcata.2014.08.015 |
| [82] | López N, Bridier B, Pérez-Ramírez J. Discriminating reasons for selectivity enhancement of CO in alkyne hydrogenation on palladium[J]. The Journal of Physical Chemistry C, 2008, 112(25): 9346–9350. DOI: 10.1021/jp711258q |
| [83] | Boitiaux J P, Cosyn J, Rbert E. Additive effects in the selective hydrogenation of unsaturated hydrocarbons on platinum and rhodium catalysts: Ⅰ: Influence of nitrogen-containing compounds[J]. Applied Catalysis, 1989, 49(2): 219–234. DOI: 10.1016/S0166-9834(00)83019-5 |
| [84] | McKenna F M, Anderson J A. Selectivity enhancement in acetylene hydrogenation over diphenyl sulphide-modified Pd/TiO2 catalysts[J]. Journal of Catalysis, 2011, 281(2): 231–240. DOI: 10.1016/j.jcat.2011.05.003 |
| [85] | McCue A J, Anderson J A. Sulfur as a catalyst promoter or selectivity modifier in heterogeneous catalysis[J]. Catalysis Science & Technology, 2014, 4(2): 272–294. |
| [86] | McCue A J, McKenna F M, Anderson J A. Triphenylphosphine: A ligand for heterogeneous catalysis too? Selectivity enhancement in acetylene hydrogenation over modified Pd/TiO2 catalyst[J]. Catalysis Science & Technology, 2015, 5(4): 2449–2459. |
 2017, Vol. 34
2017, Vol. 34