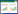2. 天津化学化工协同创新中心, 天津 300072
2. Collaborative Innovation Center of Chemical Science and Engineering, Tianjin 300072, China
有机电致发光二极管(organic light-emitting diode,OLED)作为一种新型的平板显示器件,由于其突出的优点,如自发光、反应时间快、高亮度、高效率、可弯曲、使用温度范围大而得到广泛的关注,在手机、电视机、车载显示、笔记本电脑、数码相机以及军事领域具有潜在的应用。OLED应用在照明器件中具有轻、薄、光源分布均匀、可弯曲、多彩等特点,同样具有广阔的应用前景。
在电场激发下,单重态激子与三重态激子的比值为1:3[1]。在传统的荧光材料中,电子由三重态能级到基态的辐射跃迁是禁阻的,三重态能量以非辐射跃迁的形式失活,使得器件的内部量子效率只有25%[2],外部量子效率最高为5%。相比之下,磷光材料由于利用了自旋轨道耦合效应,可以实现器件内部量子效率为100%[3]。但是磷光材料通常因为含有Ir、Pt、Os等贵金属元素而限制了其应用。最近,通过利用热活性型延迟荧光(thermally activated delayed fluorescence, TADF)材料来提高器件效率得到了广泛的关注,材料中并不含贵金属但依然利用了三重态激子,理论上内部量子效率可以达到100%。
1 热活性型延迟荧光的发光机理图 1为在光激发下发射TADF的分子能级图。由于第一激发三重态到基态的辐射跃迁是禁阻的,所以分子不能发出磷光(phosphorescence),而以非辐射跃迁的方式失活。当材料的第一激发单重态(S1)与第一激发三重态(T1)能级的能量差ΔEST接近于0,电子可以由T1能级反转系间跨越(reverse intersystem crossing, RISC)到S1能级而发出荧光,由于发生了从T1能级的反转系间跨越,所以荧光寿命比较长。不同温度下的瞬态发光衰减曲线包括2个组分,快速组分和延迟组分,寿命分别处于纳秒级和微秒级水平。研究表明延迟组分的荧光量子产率会随温度升高而升高,因此被称为热活性型延迟荧光。
|
| 图 1 在光激发下的TADF材料的分子能级图 Figure 1 The energy level diagram of TADF molecule under the light excitation |
| |
TADF材料的难点在于分子的设计[4]。一方面,TADF分子要具有小的ΔEST,降低从T1能级到S1能级RISC的势垒。另一方面,分子要具有较高的辐射衰减常数,减少非辐射跃迁能量损失。虽然增加分子的共轭程度,可以提高分子的发光效率,但同时也会提高分子的ΔEST。在分子中引入给体和受体基团,减少分子的最高占有分子轨道(the highest occupied molecular orbital, HOMO)与最低未占有分子轨道(the lowest unoccupied molecular orbital, LUMO)的重叠,从而降低分子的ΔEST[4]。
2 热活性型延迟荧光材料根据TADF材料的化学结构可分为二氰基苯咔唑类化合物、均三嗪类化合物、螺环类化合物、二苯基砜类化合物、1, 4-二氮杂菲类化合物和唑类化合物。
2.1 二氰基苯咔唑类化合物二氰基苯咔唑类化合物的分子结构式如图 2所示。量子化学计算表明咔唑基团由于空间位阻与二氰基苯平面具有较大的二面角,HOMO与LUMO分别分布在咔唑基团和二氰基苯上,因而分子具有小的ΔEST。氰基基团抑制了化合物的非辐射跃迁和激发态构型的改变,HOMO和LUMO在中间的苯环有适度重叠,使得化合物具有较高的发光效率。通过调节咔唑的个数以及在咔唑基团上引入取代基可以调节化合物的发光颜色。表 1列举了二氰基苯咔唑类化合物的TADF特性及器件参数。

|
| 图 2 二氰基苯咔唑类化合物的分子结构式 Figure 2 The molecular structures of carbazolyl dicyanobenzene compounds |
| |
| Compound | ΔEST/eV | ΦPL | Colour | EQE/% | Ref |
| 2CzPN | 0.47 a | sky-blue | 8.0 | [7] | |
| 4CzTPN-Ph | 0.26 a | orange | 11.2 | [7] | |
| 4CzIPN | ~0.08 | 0.83 | green | 19.3 | [7] |
| 4CzIPN-Me | 0.05 | 0.74 | (0.37, 0.58) b | 21.0 | [8] |
| 4CzTPN-Me | 0.67 a | (0.29, 0.57) | 19.6 | [9] | |
| 4CzTPN-t-Bu | 0.78 a | (0.31, 0.59) | 17.1 | [9] | |
| DCzIPN | 0.05 | 0.67 a | (0.17, 0.19) c | 16.4 | [10] |
| DDCzIPN | 0.13 | 0.91 a | (0.22, 0.46) c | 18.9 | [10] |
| 35IPNDCz | 0.14 | 0.58 | blue-green | 9.2 | [11] |
| 26IPNDCz | 0.06 | 0.72 | blue-green | 9.6 | [11] |
| 注:a在溶液中测得;b在500 cd/m2测得;c在1 000 cd/m2测得。 | |||||
4CzIPN是一种发绿光的TADF材料,掺杂薄膜具有较高的荧光量子产率ΦPL=0.83,ΔEST=~0.08 eV。采用器件结构:ITO/α-NPD (35 nm)/5±1%(质量分数) 4CzIPN:CBP (15 nm)/TPBi(65 nm)/LiF(0.8 nm)/Al(80 nm),器件的外部量子效率(external quantum efficiency, EQE)为19.3%。光的取出效率大致为0.2[5-6],器件内部有近100%的内部量子效率。
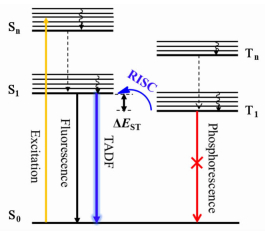
为了降低发光材料的浓度淬灭效应,提高器件的发光效率,一般将发光材料以一定的浓度掺杂在主体材料中,因此主体材料的性能对器件的效率有重要的影响。TADF主体材料要具有比发光材料更高的三重态能量(ET)、适当的HOMO和LUMO能级、较高的玻璃化转变温度(Tg)、良好的成膜能力和双极性传输性质。图 3列举了一些可作为4CzIPN的主体材料及其相应的Tg、ET和HOMO/LUMO能级。表 2总结了以4CzIPN为发光材料采用不同发光层(EML)结构的绿光(Green)和白光(White)器件的性能参数。这些器件的性能可以与磷光器件相媲美[12-13],说明TADF材料是一种非常有前景的发光材料。
|
| 图 3 4CzIPN的主体材料结构式及其性质参数 Figure 3 The molecular structures of some host materials for 4CzIPN and corresponding parameters |
| |
| Device | EML | CIE | EQE/% | Ref |
| 5% 4CzIPN: CBP | 16.0 b | [15] | ||
| 1% 4CzIPN: 3TPAPFP | (0.19, 0.43) a | 21.2 | [16] | |
| 3% 4CzIPN: PzCz | (0.20, 0.54) | 18.2 | [17] | |
| Green | 2% 4CzIPN: PPO27 | (0.20, 0.48) b | 24.2 | [18] |
| 5% 4CzIPN: mCPSOB | 26.5 c | [19] | ||
| 3% 4CzIPN: CPCB | 14.5 | [20] | ||
| 3% 4CzIPN: (mCP:BmPyPb=1:1) | (0.21, 0.53) | 28.6 | [21] | |
| 5% FIrpic:1% 4CzIPN: mCP/3% Ir(pq)2acac: TPBI | (0.49, 0.41) | 20.2 | [22] | |
| White | 15% FIrpic:1% Ir(pq)2acac: mCP/1% 4CzIPN: TPBI | (0.39, 0.46) | 18.0 | [23] |
| 10% 4CzPN: mCBP/6% 4CzPN: 2% 4CzTPN-Ph: mCBP/10% 3CzTRZ: PPT | (0.30, 0.38) | 17.1 | [24] | |
| 注:a在500 cd/m2测得;b在100 cd/m2测得;c在10 cd/m2测得。 | ||||
Nakanotani等[14]研究了TADF器件的寿命和稳定性。在组装器件时,通过测试4CzIPN掺杂在不同主体材料和载流子传输材料中薄膜的荧光量子产率,确定了最适宜的主体材料和传输材料。优化的器件结构:ITO/HATCN(10 nm)/Tris-PCz(30 nm)/4CzIPN:mCBP/T2T(10 nm)/BPy-TP2(40 nm)/LiF (0.8 nm)/Al(100 nm)。研究表明器件的使用寿命和稳定性与发光层的浓度密切相关,这是因为随着掺杂浓度的改变,电子和空穴注入的平衡性发生了变化。当掺杂质量分数为3%时,在1 000 cd/m2,器件外部量子效率为13.4%,电流效率为41.4 cd/A,半寿命为506 h。当掺杂质量分数为15%时,在1 000 cd/m2,器件外部量子效率为13.9%,电流效率为46.5 cd/A,半寿命为2 800 h。在500 cd/m2,器件的半寿命可达10 000 h。较高的掺杂浓度增加了发光层中激子形成位点,因而器件相对稳定。除此之外,在发光层和电子阻挡层的界面减少空穴的累积、减少主体分子激发态的形成、增加4CzIPN的电荷捕获能力同样可以提高器件的稳定性和寿命。
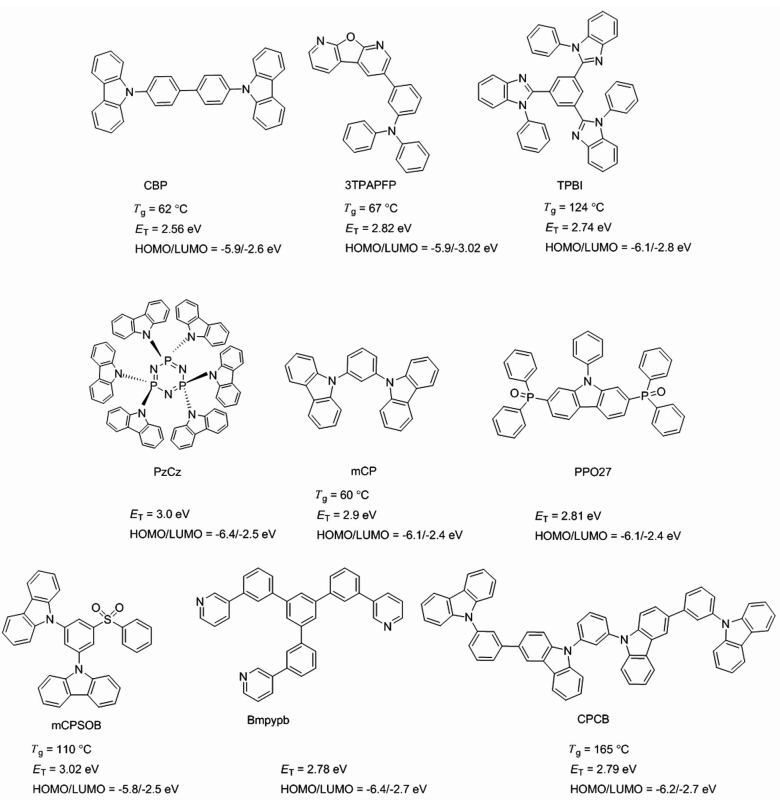
2.2 均三嗪类化合物均三嗪基团具有较强的吸电子能力而被广泛用作TADF材料的受体基团,且可在其2、4、6位进行不同取代基的修饰,增加了分子设计的多样性。给体基团可以为咔唑及其衍生物、吩恶嗪、吩噻嗪、三芳胺等。均三嗪类TADF材料的分子结构式如图 4所示,TADF特性及器件性能如表 3所示。
|
| 图 4 均三嗪类化合物的分子结构式 Figure 4 The molecular structures of s-triazine compounds |
| |
| Compound | ΔEST /eV | ΦPL | Colour | EQE/% | Ref |
| CC2TA | 0.06a | 0.62 | sky-blue | 11.0 | [25] |
| PIC-TRZ | 0.11 | 0.39 | blue-green | 5.3 | [26] |
| PIC-TRZ2 | 0.02 | 0.45 | blue-green | 14.0 | [27] |
| CzT | 0.09 | ~0.40 | blue-green | 6.0 | [28] |
| DPA-TRZ | 0.11 | 1.00 | green | 13.8 | [29] |
| DCzTRZ | 0.25 | 0.43 | (0.15, 0.16) | 17.8 | [30] |
| DDCzTRZ | 0.27 | 0.66 | (0.16, 0.22) | 18.9 | [30] |
| PXZ-TRZ | 0.07a | ~0.66 | green | 12.5 | [31] |
| PTZ-TRZ | ~0 | 532/393 nm | 10.8 | [32] | |
| bis-PXZ-TRZ | 0.05a | 0.64 | yellow-orange | 9.1 | [33] |
| tri-PXZ-TRZ | 0.06a | 0.58 | yellow-orange | 13.3 | [33] |
| 注:a由量子化学计算得到。 | |||||
Lee等[25]报道了一种可发射天蓝光的TADF材料CC2TA,由于引入的二咔唑基团增加了分子的空间位阻,导致分子的HOMO与LUMO空间重叠较小。理论计算ΔEST=0.06 eV。6% CC2TA:DPEPO薄膜的荧光量子产率为0.62。为了扩大激子的复合区域采用双发光层,器件结构:ITO/α-NPD(40 nm)/6%CC2TA:mCP (10 nm)/6%CC2TA:DPEPO (20 nm)/DPEPO(10 nm)/TPBi(30 nm)/LiF(0.8 nm)/Al(80 nm)。器件的最高外部量子效率为11%,与理论计算的外部量子效率相一致。具有相似结构的化合物如PIC-TRZ[26]、PIC-TRZ2[27]、CzT[28]、DPA-TRZ[29]、DCzTRZ[30]、DDCzTRZ[30]。
采用扭曲的给体-受体(D-A)结构同样可以打断分子的π共轭,降低分子的ΔEST。以吩恶嗪为给体、均三嗪为受体的PXZ-TRZ[31],经过X射线晶体结构分析可知由于氢原子的空间排斥作用,给体和受体二面角为74.9°,使得HOMO和LUMO在空间分离,量子化学计算的ΔEST接近于0。器件结构:ITO/α-NPD(35 nm)/6%PXZ-TRZ:CBP(15 nm)/TPBi(65 nm)/LiF(0.8 nm)/Al(80 nm)。器件发出绿光,最大发光波长为529 nm,半峰宽为92 nm,外部量子效率高达12.5%。具有相似结构的化合物如PTZ-TRZ[32],bis-PXZ-TRZ[33]、tri-PXZ-TRZ[33]。
2.3 螺环类化合物螺环化合物是一种潜在的TADF材料,分子结构式如图 5所示。这是因为螺环的结构可将给电子基团和受电子基团正交连接到π-共轭体系,降低HOMO与LUMO的空间重叠,因此可以最小化ΔEST。且在固态薄膜中可阻碍分子之间的密堆积,提高化合物的成膜性和形态稳定性。
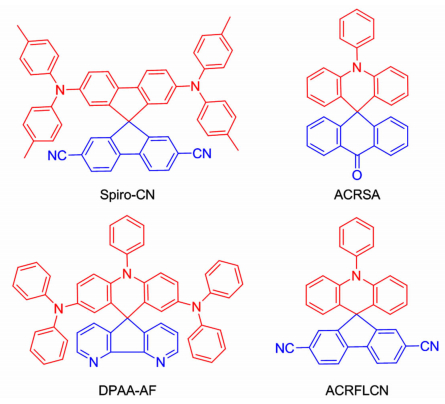
|
| 图 5 螺环化合物的分子结构式 Figure 5 The molecular structures of spiro compounds |
| |
Spiro-CN是一种发射黄光的TADF化合物,以二对甲基苯氨基基团作为供电子基团,氰基作为受电子基团[34]。由Berberan-Santos方程计算的ΔEST =0.057eV,荧光量子产率为0.27。采用器件结构:ITO/α-NPD (60 nm)/6% Spiro-CN:mCP(20 nm)/Bphen(40 nm)/MgAg(100 nm)/Ag (20 nm)。器件的最大电流效率为13.5 cd/A,最高外部量子效率为4.4%。具有相似结构的化合物如ACRFLCN[35]、ACRSA[36]、DPAA-AF[37],器件的最大外部量子效率分别为10.1%、16.5%、9.6%。
2.4 二苯基砜类化合物激发过程中的电荷转移(charge transfer, CT)使得单重电荷转移态(1CT)和三重电荷转移态(3CT)之间具有小的ΔEST,因此可以实现电荷转移态的热活性型延迟荧光。一般而言,芳香族化合物的本征激发态(locally excited, LE)是最低三重态(3LE),所以从T1态到S1态的RISC过程包括从3LE(如3ππ*态)反内转换(reverse internal conversion, RIC)到3CT态,再反系间跨越到1CT态,从而实现TADF的发射,如图 6所示。因而只有当化合物的3LE态接近或高于3CT态,单重态和三重态之间的能量交换才能有效地进行。所以在设计光学带隙较宽的蓝光TADF材料时,应考虑到给电子基团与受电子基团的π共轭程度及氧化还原电位,同时打断给受体基团之间的共轭。一些以二苯基砜类为受体的化合物可发射高效的TADF蓝光,分子结构式如图 7所示。

|
| 图 6 T1态到S1态的反系间跨越(RISC)过程示意图 Figure 6 The schematic diagram of RISC process from T1 state to S1 state |
| |

|
| 图 7 二苯基砜类化合物的结构式 Figure 7 The molecular structures of diphenylsulphone compounds |
| |
Zhang等报道了蓝光TADF化合物DPA-DPS[38]、DTA-DPS[38]及DTC-DPS[38]。以DPEPO为主体材料,共掺薄膜的最大发射波长分别在421、430和423 nm,荧光量子产率分别为0.60、0.66和0.80。ΔEST分别为0.54、0.45和0.32 eV。采用器件结构:ITO/α-NPD (30 nm)/TCTA (20 nm)/CzSi (10 nm)/EML (20 nm)/DPEPO (10 nm)/TPBI(30 nm)/LiF (1 nm)/Al,器件的最大外部量子效率分别为2.9%、5.6%和9.9%。以DTC-DPS为发光材料的CIE坐标为(0.15,0.07),非常接近标准蓝色坐标(0.14,0.08)。但由于较长的激子寿命和较大的ΔEST,器件存在严重的“效率滚降”。通过提高给体基团的给电子能力可降低此类化合物的ΔEST。以3, 6-二甲氧基-咔唑为给体,二苯基砜基团为受体的TADF化合物DMOC-DPS[39],ΔEST为0.21 eV,比DTC-DPS减小了0.11 eV,同时激子的寿命也降低到127 μs。采用上述器件结构,器件最大电致发光波长在460 nm,CIE坐标(0.16,0.16),最大外部量子效率为14.5%,“效率滚降”相比DTC-DPS器件也轻微了很多。
PXZ-DPS[40]是另一种性能较好的TADF蓝光材料。量子化学计算表明PXZ与相连的苯环之间的二面角为80°,较大的扭曲角降低了分子的共轭,使得HOMO和LUMO空间重叠较少,ΔEST为0.08 eV。掺杂薄膜的荧光量子产率为0.9,采用器件结构:ITO/α-NPD(40 nm)/10%PXZ-DPS:CBP (20 nm)/TPBI(60 nm)/LiF (1 nm)/Al,器件的启亮电压在2.7 V,最大外部量子效率为17.5%。以DMAC-DPS作为TADF材料的器件最高外部量子效率为19.5%,CIE坐标(0.16,0.20),与以FIrpic作为发光材料的磷光器件相比,效率相近,但颜色纯度更佳。
2.5 1, 4-二氮杂菲类化合物1, 4-二氮杂菲包含3个稠合的苯环和氮杂环,因而具有较大的共轭面积和吸电子能力。Takahashi等分别以吩口恶嗪,9, 9-二甲基吖啶和3-(二苯基氨基)-咔唑为给体,通过在1, 4-二氮杂菲基团上的7、10位或者6、11位进行取代报道了一系列1, 4-二氮杂菲类TADF材料[41],材料结构式如图 8所示。研究表明化合物的ΔEST在0.04~0.26 eV,掺杂薄膜的荧光量子产率在0.49~0.81。发射绿光的ATP-PXZ、m-ATP-PXZ器件,最大外部量效率分别为11.7%、12.6%。发射天蓝光的ATP-ACR、m-ATP-ACR、m-ATP-CDP器件最大外部量效率分别为7.5%、8.7%、7.5%。对于相同的给体和受体基团,取代位置的不同对器件的性能影响不是很大。
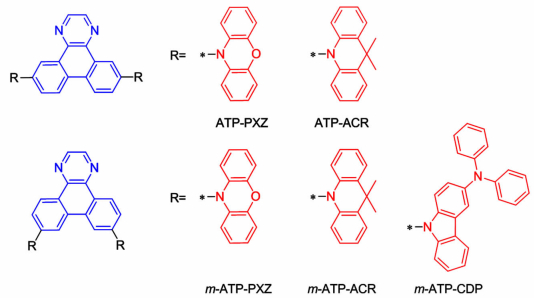
|
| 图 8 1, 4-二氮杂菲化合物的分子结构式 Figure 8 The molecular structures of 1, 4-diazatriphenylene compounds |
| |
唑类化合物由于具有较强的吸电子能力而被广泛应用为电子传输材料,如OXD-7[42],t-Bu-Taz[43]。以三唑(TAZ)、噁二唑(OXD)、噻二唑(TDZ)为受体,吩恶嗪为给体的TADF材料具有较高的器件效率。2PXZ-TAZ[44]、2PXZ-OXD[44]和2PXZ-TDZ[45]的结构式如图 9所示。

|
| 图 9 唑类化合物的分子结构式 Figure 9 The molecular structures of azole compounds |
| |
通过改变中心原子实现对化合物的光色调节。研究表明随着中心原子的原子序数增加(N、O、S),化合物的共轭程度增加,荧光光谱发生红移。ΔEST也依次降低,分别为0.23、0.15和0.11 eV。采用器件结构:ITO/α-NPD (30 nm)/mCP (10 nm)/EML (15 nm)/DPEPO (10 nm)/TPBi (40 nm)/LiF (0.8 nm)/Al (100 nm),器件分别发出天蓝光、绿光和橙色光,最大外部量子效率分别为6.4%、14.9%和10%。
3 结论与展望在电致发光器件中,热活性型延迟荧光材料利用三重态和单重态激子,理论上内部量子效率可达到100%,已经成为当前研究的热点。此外,材料成本低、易于制备,有望成为下一代发光材料,这将推动OLED市场的快速发展。
为了实现高效的热活性型延迟荧光,分子要满足较小的ΔEST和较高的荧光量子产率。通过在分子中引入给体和受体基团可实现高效的电荷转移态的延迟荧光。在分子中引入大的取代基、设计扭曲的分子结构以及螺环结构都有利于降低分子的ΔEST。选择稠环化合物作为给体或者受体有利于提高分子的荧光量子产率。选择不同的给体或者受体基团、调节给体/受体比例、取代基修饰等成为设计TADF分子的重要手段。
| [1] | Segal M, Baldo M A, Holmes R J, et al. Excitonic singlet-triplet ratios in molecular and polymeric organic materials[J]. Physical Review B, 2003, 68(7): 338–344. |
| [2] | Rothberg L J, Lovinger A J. Status of and prospects for organic electroluminescence[J]. Journal of Materials Research, 1996, 11(12): 3174–3187. DOI: 10.1557/JMR.1996.0403 |
| [3] | Adachi C, Baldo M A, Thompson M E, et al. Nearly 100% internal phosphorescence efficiency in an organic light-emitting device[J]. Journal of Applied Physics, 2001, 90(10): 5048–5051. DOI: 10.1063/1.1409582 |
| [4] | Adachi C. Third-Generation organic electroluminescence materials[J]. Japanese Journal of Applied Physics, 2014, 53(6): 060101. DOI: 10.7567/JJAP.53.060101 |
| [5] | Smith L H, Wasey J A E, Barnes W L. Light outcoupling efficiency of top-emitting organic light-emitting diodes[J]. Applied Physics Letters, 2004, 84(16): 2986–2988. DOI: 10.1063/1.1712036 |
| [6] | Madigan C F, Lu M H, Sturm J C. Improvement of output coupling efficiency of organic light-emitting diodes by backside substrate modification[J]. Applied Physics Letters, 2000, 76(13): 1650–1652. DOI: 10.1063/1.126124 |
| [7] | Uoyama H, Goushi K, Shizu K, et al. Highly efficient organic light-emitting diodes from delayed fluorescence[J]. Nature, 2012, 492(7 428): 234–238. |
| [8] | Furukawa T, Nakanotani H, Inoue M, et al. Dual enhancement of electroluminescence efficiency and operational stability by rapid upconversion of triplet excitons in OLEDs[J]. Scientific Reports, 2015, 5: 8429. DOI: 10.1038/srep08429 |
| [9] | Cho Y J, Yook K S, Lee J Y. High efficiency in a solution-processed thermally activated delayed-fluorescence device using a delayed-fluorescence emitting material with improved solubility[J]. Advanced Materials, 2014, 26(38): 6642–6646. DOI: 10.1002/adma.v26.38 |
| [10] | Cho Y J, Jeon S K, Chin B D, et al. The design of dual emitting cores for green thermally activated delayed fluorescent materials[J]. Angewandte Chemie International Edition, 2015, 54(17): 5201–5204. DOI: 10.1002/anie.v54.17 |
| [11] | Li B, Nomura H, Miyazaki H, et al. Dicarbazolyldicyanobenzenes as thermally activated delayed fluorescence emitters: Effect of substitution position on photoluminescent and electroluminescent properties[J]. Chemistry Letters, 2014, 43(3): 319–321. DOI: 10.1246/cl.130907 |
| [12] | Hung W, Chi L, Chen W, et al. A carbazole-phenylbenzimidazole hybrid bipolar universal host for high efficiency RGB and white PhOLEDs with high chromatic stability[J]. Journal of Materials Chemistry, 2011, 21(48): 19249–19256. DOI: 10.1039/c1jm14029a |
| [13] | Lin W, Huang W, Huang M, et al. A bipolar host containing carbazole/dibenzothiophene for efficient solution-processed blue and white phosphorescent OLEDs[J]. Journal of Materials Chemistry C, 2013, 1(41): 6835–6841. DOI: 10.1039/c3tc31357c |
| [14] | Nakanotani H, Masui K, Nishide J, et al. Promising operational stability of high-efficiency organic light-emitting diodes based on thermally activated delayed fluorescence[J]. Scientific Reports, 2013, 3: 2127. DOI: 10.1038/srep02127 |
| [15] | Komatsu R, Sasabe H, Inomata S, et al. High efficiency solution processed OLEDs using a thermally activated delayed fluorescence emitter[J]. Synthetic Metals, 2015, 202: 165–168. DOI: 10.1016/j.synthmet.2015.02.009 |
| [16] | Im Y, Lee J Y. Above 20% external quantum efficiency in thermally activated delayed fluorescence device using furodipyridine-type host materials[J]. Chemistry of Materials, 2014, 26(3): 1413–1419. DOI: 10.1021/cm403358h |
| [17] | Nishimoto T, Yasuda T, Lee S Y, et al. A six-carbazole-decorated cyclophosphazene as a host with high triplet energy to realize efficient delayed-fluorescence OLEDs[J]. Materials Horizons, 2014, 1(2): 264–269. DOI: 10.1039/C3MH00079F |
| [18] | Kim B S, Lee J Y. Phosphine oxide type bipolar host material for high quantum efficiency in thermally activated delayed fluorescent device[J]. ACS Applied Materials & Interfaces, 2014, 6(11): 8396–8400. |
| [19] | Gaj M P, Hernandez C F, Zhang Y, et al. Highly efficient organic light-emitting diodes from thermally activated delayed fluorescence using a sulfone-carbazole host material[J]. Organic Electronics, 2015, 16: 109–112. DOI: 10.1016/j.orgel.2014.10.049 |
| [20] | Suzuki Y, Zhang Q, Adachi C. A solution-processable host material of 1, 3-bis{3-[3-(9-carbazolyl)phenyl]-9-carbazolyl}benzene and its application in organic light-emitting diodes employing thermally activated delayed fluorescence[J]. Journal of Materials Chemistry C, 2015, 3(8): 1700–1706. DOI: 10.1039/C4TC02211D |
| [21] | Kim B S, Lee J Y. Engineering of mixed host for high external quantum efficiency above 25% in green thermally activated delayed fluorescence device[J]. Advanced Functional Materials, 2014, 24(25): 3970–3977. DOI: 10.1002/adfm.v24.25 |
| [22] | Kim B S, Yook K S, Lee J Y. Above 20% external quantum efficiency in novel hybrid white organic light-emitting diodes having green thermally activated delayed fluorescent emitter[J]. Scientific Reports, 2014, 4: 6019. DOI: 10.1038/srep06019 |
| [23] | Kim B S, Lee J Y. Interlayer free hybrid white organic light-emitting diodes with red/blue phosphorescent emitters and a green thermally activated delayed fluorescent emitter[J]. Organic Electronics, 2015, 21: 100–105. DOI: 10.1016/j.orgel.2015.02.022 |
| [24] | Nishide J, Nakanotani H, Hiraga Y, et al. High-Efficiency white organic light-emitting diodes using thermally activated delayed fluorescence[J]. Applied Physics Letters, 2014, 104(23): 233304. DOI: 10.1063/1.4882456 |
| [25] | Lee S Y, Yasuda T, Nomura H, et al. High-Efficiency organic light-emitting diodes utilizing thermally activated delayed fluorescence from triazine-based donor-acceptor hybrid molecules[J]. Applied Physics Letters, 2012, 101(9): 1585. |
| [26] | Endo A, Sato K, Yoshimura K, et al. Efficient up-conversion of triplet excitons into a singlet state and its application for organic light emitting diodes[J]. Applied Physics Letters, 2011, 98(8): 42. DOI: 10.1063/1.3558906 |
| [27] | Sato K, Shizu K, Yoshimura K, et al. Organic luminescent molecule with energetically equivalent singlet and triplet excited states for organic light-emitting diodes[J]. Physical Review Letters, 2013, 110(24): 247401–247401. DOI: 10.1103/PhysRevLett.110.247401 |
| [28] | Sereviččius T, Nakagawa T, Kuo M C, et al. Enhanced electroluminescence based on thermally activated delayed fluorescence from a carbazole-triazine derivative[J]. Physical Chemistry Chemical Physics, 2013, 15(38): 15850–15855. DOI: 10.1039/c3cp52255e |
| [29] | Shizu K, Uejima M, Nomura H, et al. Enhanced electroluminescence from a thermally activated delayed-fluorescence emitter by suppressing nonradiative decay[J]. Physical Review Applied, 2015, 3(1): 014001. DOI: 10.1103/PhysRevApplied.3.014001 |
| [30] | Kim M, Jeon S K, Hwang S H, et al. Stable blue thermally activated delayed fluorescent organic light-emitting diodes with three times longer lifetime than phosphorescent organic light-emitting diodes[J]. Advanced Materials, 2015, 27(15): 2515–2520. DOI: 10.1002/adma.201500267 |
| [31] | Tanaka H, Shizu K, Miyazakiab H, et al. Efficient green thermally activated delayed fluorescence (TADF) from a phenoxazine-triphenyltriazine (PXZ-TRZ) derivative[J]. Chemical Communications, 2012, 48(93): 11392–11394. DOI: 10.1039/c2cc36237f |
| [32] | Tanaka H, Shizu K, Nakanotani H, et al. Dual intramolecular charge-transfer fluorescence derived from a phenothiazine-triphenyltriazine derivative[J]. The Journal of Physical Chemistry C, 2014, 118(29): 15985–15994. DOI: 10.1021/jp501017f |
| [33] | Tanaka H, Shizu K, Nakanotani H, et al. Twisted intramolecular charge transfer state for long-wavelength thermally activated delayed fluorescence[J]. Chemistry of Materials, 2013, 25(18): 3766–3771. DOI: 10.1021/cm402428a |
| [34] | Nakagawa T, Ku S Y, Wong K T, et al. Electroluminescence based on thermally activated delayed fluorescence generated by a spirobifluorene donor-acceptor structure[J]. Chemical Communications, 2012, 48(77): 9580–9582. DOI: 10.1039/c2cc31468a |
| [35] | Méhes G, Nomura H, Zhang Q, et al. Enhanced electroluminescence efficiency in a spiro-acridine derivative through thermally activated delayed fluorescence[J]. Angewandte Chemie International Edition, 2012, 51(45): 11311–11315. DOI: 10.1002/anie.201206289 |
| [36] | Nasu K, Nakagawa T, Nomura H, et al. A highly luminescent spiro-anthracenone-based organic light-emitting diode exhibiting thermally activated delayed fluorescence[J]. Chemical Communications, 2013, 49(88): 10385–10387. DOI: 10.1039/c3cc44179b |
| [37] | Ohkuma H, Nakagawa T, Shizu K, et al. Thermally activated delayed fluorescence from a spiro-diazafluorene derivative[J]. Chemistry Letters, 2014, 43(7): 1017–1019. DOI: 10.1246/cl.140360 |
| [38] | Zhang Q, Li J, Shizu K, et al. Design of efficient thermally activated delayed fluorescence materials for pure blue organic light emitting diodes[J]. Journal of the American Chemical Society, 2012, 134(36): 14706–14709. DOI: 10.1021/ja306538w |
| [39] | Wu S, Aonuma M, Zhang Q, et al. High-Efficiency deep-blue organic light-emitting diodes based on a thermally activated delayed fluorescence emitter[J]. Journal of Materials Chemistry C, 2013, 2(3): 421–424. |
| [40] | Zhang Q, Li B, Huang S, et al. Efficient blue organic light-emitting diodes employing thermally activated delayed fluorescence[J]. Nature Photonics, 2014, 8(4): 326–332. DOI: 10.1038/nphoton.2014.12 |
| [41] | Takahashi T, Shizu K, Yasuda T, et al. Donor-Acceptor-structured 1, 4-diazatriphenylene derivatives exhibiting thermally activated delayed fluorescence: Design and synthesis, photophysical properties and OLED characteristics[J]. Science and Technology of Advanced Materials, 2014, 15(3): 034202. DOI: 10.1088/1468-6996/15/3/034202 |
| [42] | Lee J, Chopra N, Eom S H, et al. Effects of triplet energies and transporting properties of carrier transporting materials on blue phosphorescent organic light emitting devices[J]. Applied Physics Letters, 2008, 93: 123306. DOI: 10.1063/1.2978235 |
| [43] | Wang Q, Ding J, Ma D, et al. Harvesting excitons via two parallel channels for efficient white organic LEDs with nearly 100% internal quantum efficiency: Fabrication and emission-mechanism analysis[J]. Advanced Functional Materials, 2009, 19(1): 84–95. DOI: 10.1002/adfm.v19:1 |
| [44] | Lee J, Shizu K, Tanaka H, et al. Oxadiazole-and triazole-based highly-efficient thermally activated delayed fluorescence emitters for organic light-emitting diodes[J]. Journal of Materials Chemistry C, 2013, 1(30): 4599–4604. DOI: 10.1039/c3tc30699b |
| [45] | Tanaka H, Shizu K, Lee J, et al. Effect of atom substitution in chalcogenodiazole-containing thermally activated delayed fluorescence emitters on radiationless transition[J]. The Journal of Physical Chemistry C, 2015, 119: 2948–2955. DOI: 10.1021/jp510751n |
 2017, Vol. 34
2017, Vol. 34