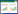China is the largest coke producer,exporter and consumer in the world[1],and the coking industry has become an important part of China′s national economy[2]. The coke oven,with the combustion chamber as its heating system,is the main equipment of coke production. When the coke oven is in operation,the heat,coming from the combustion between fuel gases and preheated air in the combustion chamber,is transferred to the coal on the other side of the furnace wall by convection and radiation. During combustion,a certain amount of nitrogen oxides (NOx) is produced[3],which results in atmospheric pollution to destroy the ozone layer[4],cause acid rain[5] and affect the health of human being[6]. It is reported that about 67%[7] of the NOx in China comes from coal chemical industry. In order to reduce NOx emission,more rigorous emission standards of NOx in coal chemical industry have been promulgated in recent years. So,carrying out research on NOx emission produced by coal chemical industry is warranted.
Research on NOx emission in coal chemical industry has been conducted by many researchers. Fu[8] discussed NOx formation mechanisms in the process of coal combustion. Jin[9] and Choi[10] carried out research on NOx release characteristics in pulverized-coal fired boilers. In the work of Liu[11],the process simulation of formation and emission of NOx during coal decoupling combustion was conducted. Further,a large number of research efforts are ongoing with the aim of reducing NOx emission in coal chemical industry. Technologies,i.e. oxy-fuel combustion[12],over fire air combustion[13],air staging technology[14],combustors design[15],have been used to reduce NOx. Although considerable work has been done relating to NOx emission in coal chemical industry,a limited number of studies address NOx formation in coke oven combustion chambers.
Therefore,the objective of this study is to investigate NOx formation in a coke oven combustion chamber. Since the high temperature distributed in the combustion chamber,conducting this research by traditional ways is difficult. Numerical simulation methods,being able to reveal detail in high temperature[16],have been applied by this study. In this paper,the distribution of NOx in the combustion chamber is presented,which provides detailed information of NOx behavior in the combustion chamber. In addition,the effects of geometry of the combustion chamber and air preheated temperature on NOx formation are discussed as well.
1 Computational domainThe coke oven combustion chamber is divided into many vertical flues by furnace walls. In this paper,only adjacent two vertical flues,forming one twin flue,are studied,which are 3.500,0.175,0.493 m in height,length and width,repectively. The two flues,acted as upward vertical flue and downward vertical flue repectively,connect with each other by the cross hole on the top and two loop holes at the bttom. The nozzles at the bottom of upward vertical flue and downward vertical flue act as inlets for air and outlets for exhaust gas repectively. As heating gas,the coke oven gas (COG),whoes compositions are shown in Table 1,is input from the brick gas port in the bottom of upward vertical flue. The whole geometry of the coke oven combustion chamber is shown in Fig. 1. About 150,000 hexahedral grids,which were choosed by the consideration of accurate simulated results and economic simulated time in the grid independency test,were created in a computer aided design (CAD) program called Gambit 2.4.6 and exported into Ansys Fluent 14.0.
|
| Fig.1 The geometry of the coke oven combustion chamber |
Combustion in the coke oven combustion chamber,contained the mixing and transport of chemical species of COG and air,could be modeled by solving conservation equations to describe convection,diffusion and reaction sources for each component species. The transport equations,included mass,momentum,energy and species equations,can be typically represented by the following general form:
| $\frac{{\partial \left( {\rho \Phi } \right)}}{{\partial t}} + div\left( {\rho \vec u\Phi } \right) = div\left( {\Gamma grad\Phi } \right) + S$ | (1) |
Where Φ is the generalized variable,Γ is the generalized diffusion coefficient,S is the generalized source term. The three variables have different forms for different equations and a more detailed description of transport equations is given in literature[17].
2.2 Turbulent modelAt present,the standard k-ε model has become the workhorse of practical engineering flow calculations since its robustness,economy and reasonable accuracy for a wide range of turbulent flows[18, 19]. So the standard k-ε model with default values was adopted to model the dynamic of the turbulent flow in the combustion chamber of the coke oven. k equation and ε equation are given in formula (2) and (3),respectively.
| $\begin{array}{l} \frac{\partial }{{\partial t}}\left( {\rho k} \right) + \frac{\partial }{{\partial {x_i}}}\left( {\rho k{u_i}} \right) = \frac{\partial }{{\partial {x_j}}} = \frac{\partial }{{\partial {x_j}}}\left[{\left( {\mu + \frac{{{\mu _t}}}{{{\sigma _k}}}} \right)\frac{{\partial k}}{{\partial {x_j}}}} \right] + \\ \;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;{G_k} + {G_b} - \rho \varepsilon - {Y_M} + {S_k} \end{array}$ | (2) |
| $\begin{array}{l} \frac{\partial }{{\partial t}}\left( {\rho \varepsilon } \right) + \frac{\partial }{{\partial {x_i}}}\left( {\rho \varepsilon {u_i}} \right) = \frac{\partial }{{\partial {x_j}}} = \frac{\partial }{{\partial {x_j}}}\left[{\left( {\mu + \frac{{{\mu _t}}}{{{\sigma _\varepsilon }}}} \right)\frac{{\partial k}}{{\partial {x_j}}}} \right] + \\ {C_{1\varepsilon }}\frac{\varepsilon }{k}\left( {{G_k} + {C_{3\varepsilon }} + {G_b}} \right) - {C_{2\varepsilon }}\rho \frac{{{\varepsilon ^2}}}{k} + {S_\varepsilon } \end{array}$ | (3) |
Where,Gk represents the generation of turbulence kinetic energy due to the mean velocity gradients; Gb is the generation of turbulence kinetic energy due to buoyancy; YM represents the contribution of the fluctuating dilatation in compressible turbulence to the overall dissipation rate; C1ε,C2ε and C3ε are constants; σk and σε are the turbulent Prandtl numbers for k and ε,respectively; Sk and Sε are user-defined source terms.
2.3 Combustion modelIn this study,combustion reactions between COG and air were calculated using the eddy dissipation concept(EDC) combustion model[20, 21],which considers detailed chemical mechanisms in turbulent flows and assumes reactions occur in small turbulent structures,called the fine structure,under a steady assumption,according to the law:
| $R = \frac{{\rho {{\left( {\xi *} \right)}^2}}}{{\tau *\left[{1 - {{\left( {\xi *} \right)}^3}} \right]}}\left( {{Y_i}* - {Y_i}} \right)$ | (4) |
Where ξ*=Cξ(υε/k2)1/4 is the volume fraction of the fine structure, Cξ is a volume fraction constant whose value is 2.1377; Yi is the species mass fraction,Y*i is the fine-scale species mass fraction after reacting over the time τ*=Cτ(υ/ε)1/2,Cτ=0.4082,which is time scale constant.
Reaction starts after the time scales τ* and the reaction rate is controlled by Arrhenius equation,and the forward rate constant of the reaction is calculated through the Arrhenius formula:
| ${k_{f,r}} = {A_r}{T^{{\beta _r}}}\exp \left( { - {E_r}/RT} \right)$ | (5) |
Where Ar is pre-exponential factor; βr is temperature exponent; Er is activation energy (J·kmol-1) and R is universal gas constant (J·kmol-1·K-1).
2.4 Radiation modelDuring the combustion process of COG,the high temperature free carbon produced by pyrolysis has strong radiation ability and radiation is the dominant mode of heat transfer in the coke oven combustion chamber[22]. In this study,the discrete ordinates (DO) radiation model[23, 24],which has generally been chosen in the computational fluid dynamic(CFD) applications to simulate the industry processes because of higher accuracy[25, 26],was used with the weighted sum of gray gases model to calculate the gas mixtures absorption coefficients,whose radiative transfer equation at a position $\overrightarrow r $ in the direction $\overrightarrow s $ can be written as:
| $\begin{array}{l} \frac{{dI\left( {\overrightarrow r ,\overrightarrow s } \right)}}{{ds}} + \left( {a + {\sigma _s}} \right)I\left( {\overrightarrow r ,\overrightarrow s } \right) = a{n^2}\frac{{\sigma {T^4}}}{\pi } + \\ \frac{{{\sigma _s}}}{{4\pi }}\int\limits_0^{4\pi } I \left( {\overrightarrow r ,\overrightarrow s '} \right)\Phi \left( {\overrightarrow s \cdot \overrightarrow s '} \right)d\Omega ' \end{array}$ | (6) |
Where I is radiation intensity,σs is scattering coefficient,a is absorption coefficient,s′ is the scattering direction vector,σ is the Stefan-Boltzmann constant (5.669×10-8 W·m-2·K-4),and Ω′ is the solid angle,Φ is the phase function.
2.5 NOx modelTo predict NOx emission,Ansys Fluent solves additional transport equations for NOx concentration based on a given flow field and combustion solution. In other words,NOx is postprocessed from a combustion simulation. There are three types of mechanisms for the formation of NOx in combustion process: thermal-NOx,rapid-NOx and fuel-NOx,the NOx in coke oven combustion chambers is mainly formed in thermal-NOx[3].
Thermal-NOx mechanism arises from the thermal dissociation and subsequent reaction of nitrogen and oxygen molecules in combustion air at relatively high temperature in fuel-lean environment. Thermal-NOx mechanism is described by a set of chemical reactions known as the Zeldovich mechanism[27]:
| ${N_2} + O \leftrightarrow NO + N$ | (7) |
| $N + {O_2} \leftrightarrow NO + O$ | (8) |
Using these two reactions,the net rate of NO formation can be calculated as:
| $\frac{{d{c_{NO}}}}{{dt}} = {k_{1f}}{c_{{N_2}}}{c_O} + {k_{2f}}{c_N}{c_{{O_2}}} - {k_{1b}}{c_{NO}}{c_N} - {k_{2b}}{c_{NO}}{c_O}$ | (9) |
Where k1f,k2f are the rate coefficients for positive reactions,while k1b,k2b are for negative reactions.
The expression of the overall rate of thermal-NOx is given by:
| $\frac{{d{c_{NO}}}}{{dt}} = 3 \times {10^{14}}{c_{{N_2}}}{c_{{O_2}}}^{\frac{1}{2}}\exp \left( { - \frac{{542000}}{{RT}}} \right)$ | (10) |
The temperature and the concentration of O2 during the combustion process are the main factors to effect the formation of thermal-NOx[28].
3 Numerical simulations 3.1 Boundary conditionsIn the study,mass flows at inlets were imposed,together with the pressure of -60 Pa at the outlet section,while the walls were adiabatic except carbonization chamber walls,which were set to a constant heat flux. The value of constant heat flux through carbonization chamber walls was obtained from the heat balance calculations of the combustion chamber.
3.2 Numerical algorithmSimulations had been carried out with a Pressure-Based solver in Ansys Fluent14.0 and the SIMPLE was used to resolve the press-velocity correcting equation. The pressure equation was discretized by standard method and the momentum equation,the energy equation were discretized by a 1st order.
3.3 Initialization methodIn order to solve the closed set of governing model equations,it is necessary to specify appropriate initial conditions. In this paper,initial values were obtained from material balance calculations.
4 Results and discussionIn the present study,six simulation cases were carried out and the detail information is listed in Table 2. In order to study the influence of exhaust gas recirculation process on the formation of NOx in the combustion chamber,the circulation ports of the geometry were cut away in case1. The reasonable range of air preheated temperature in coke oven combustion chambers,is from 1 173.15 K to 1 593.15 K[22],because the fuel gases will not be ignited under too low preheated temperature and too much energy for heating the air will be consumed in turn for air with too high preheated temperature.
| 1 | No | 1 373.15 |
| 2 | Yes | 1 173.15 |
| 3 | Yes | 1 273.15 |
| 4 | Yes | 1 373.15 |
| 5 | Yes | 1 473.15 |
| 6 | Yes | 1 573.15 |
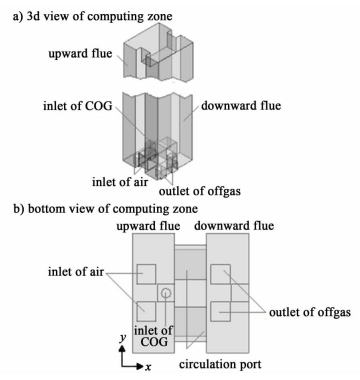
In section 4.1,only results and discussion of case4 were present since similar conclusions could be found in other cases. Fig. 2 and Fig. 3 show the NOx distribution in the combustion chamber. In the upward vertical flue,area with very low mass fraction of NOx could be found near the inlets of air and COG. Since the combustion between O2 and COG does not progress actively near the inlets of gases,the temperature of gas mixtures in this region is too low to cause the dissociation of N2 and activate the formation reactions of NOx. With the increase of the flue height,the combustion reactions between O2 and COG become intense gradually,the temperature of gas mixtures near the combustion zone increases quickly (see in Fig. 4),which is high enough to cause the formation reactions of NOx. With the happening of the combustion reactions,the temperature of gas mixtures becomes higher and more NOx is formed. At the height of 1.4 m,the NOx concentration reaches the maximum,while it decreases in the upper region of the upward vertical flue(h>1.4 m). Because of the mass consumption of O2 in the combustion reactions (see in Fig. 4),the contact of O2 and N2 drops greatly,which decreases the number of the NOx formation reactions and the NOx concentration in the upper region.

|
| Fig.2 Mass fraction of NOx in the upward flue |

|
| Fig.3 Mass fraction of NOx in the downward flue |
|
| Fig.4 The distribution of temperature, concentrations of NOx and O2 in the upward flue |
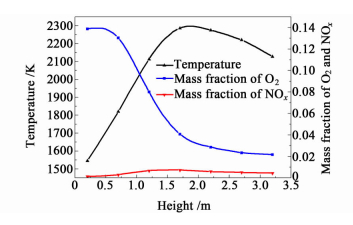
The NOx distribution in the downward vertical flue is clearly different compared to that in the upward vertical flue. In the downward vertical flue,the temperature of gas mixtures decreases with the decline of the flue height due to the absence of combustion reactions. In addition,the change of the temperature of gas mixtures does not lead to marked change of NOx mass fraction (as shown in Fig. 5),which indicates that the formation of NOx in the downward vertical flue is controlled by O2 concentration. On account of the reversible reactions of NOx formation,the mass fraction of O2 in the downward vertical flue is almost unchanged,which causes the uniform distribution of NOx concentration in the downward vertical flue.
|
| Fig.5 The distribution of temperature, concentrations of NOx and O2 in the downward flue |
Exhaust gas recirculation process is often used to reduce NOx formation in coal chemical industry. Therefore,the effect of exhaust gas recirculation process on the formation of NOx in the combustion chamber is discussed. The only difference between cases 1 and case 4 is that the combustion chamber in case4 employs the exhaust gas recirculation process.
The flow fields in the two cases are shown in Fig. 6. Similar velocity distributions in cases 1 and case 4 can be found in the upward vertical flue,and apparent difference exist in the downward vertical flue. For case 1,the gas mixtures move down continuously and all of them are discharged from the nozzles of outlets as exhaust gas,while in case 4,at the bottom zone of the downward vertical flue,only a part of the gas mixtures are discharged from the nozzles of outlets as exhaust gas,some flow into the upward vertical flue through circulation ports between the flues.

|
| Fig.6 The velocity fields in case 1 and case 4 |
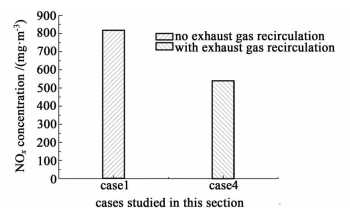
Owing to the exhaust gas recirculation process,the NOx concentration in case 4 drops by 33.8% compared to that of case 1,as shown in Fig. 7. The exhaust gas recirculation process employed in case 4 accelerates the upward flow velocity of gas mixtures in the upward vertical flue,which extends the combustion flame and causes larger combustion zone to dilute the concentration of O2 in high temperature zone. The access of gas mixtures from the downward vertical flue increases the total number of gas molecules in the upward vertical flue,which dilutes the concentration of O2 as well. On the other hand,the mix of gases from the downward vertical flue and the original gases existed in the upward vertical flue lowers the temperature of gas mixtures in the upward vertical flue. Because of the dual influence of decline of O2 concentration and temperature in the upward vertical flue,where contains the main formation area for NOx,the formation of NOx in case4 is reduced. Therefore,employing exhaust gas recirculation process in combustion chambers is an effective way to reduce NOx concentration in exhaust gas.
|
| Fig.7 The concentrations of NOx in case 1 and case 4 |
Comparison of simulated NOx concentrations in case 1 and case 4 to measured values[29] is listed in Table 3. From Table 3 we can see that the differences in term of NOx concentration between simulated results and measured values are within 10%,which confirms the reliability of simulated results in this paper well.
| Item | Simulated value/ (g·m-3) | Measured value/ (g·m-3) | Difference/ % |
| Case 1 without exhaust gas recirculation | 0.82 | 0.8~1.0 | 0 |
| Case 4 with exhaust gas recirculation | 0.54 | 0.5 | 8 |
Different coke oven has different air preheated temperature,so the effect of air preheated temperature on the formation of NOx in the coke oven combustion chamber is investigated as well.
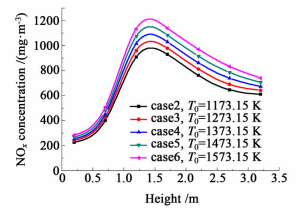
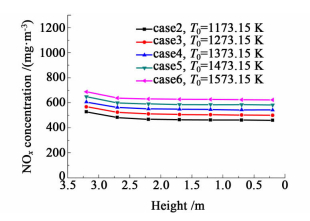
The NOx concentrations in different height of the flues in dependence of air preheated temperature are shown in Fig. 8 and Fig. 9. The NOx concentrations in all cases studied in this section have the same trend: they increase and then decrease in the upward vertical flue and decline slowly in the downward vertical flue. However,curves of cases with higher air preheated temperature are always above that of the other cases,no matter in the upward vertical flue or the downward vertical flue. The reason is that air with higher preheated temperature not only possesses higher enthalpy,but also enhances the temperature of combustion reactions in the combustion chamber. With higher combustion reaction temperature,more heat will be released by the combustion reactions and higher temperature result,which enhances the formation of NOx in the combustion chamber. In addition,narrow gaps between curves occur in the bottom region of the upward vertical flue (h<1.1 m),this is due to the fact that this region rarely has NOx formation,thus the NOx concentrations here in all cases close to each other.
|
| Fig.8 NOx concentrations about case 2~6 in upward flue |
|
| Fig.9 NOx concentrations about case 2~6 in downward flue |
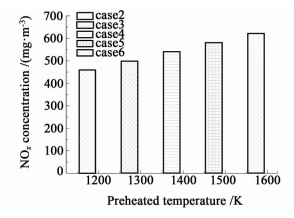
The NOx concentrations in exhaust gas of case 2~6 are shown in Fig. 10. It can be noticed that the NOx concentration in exhaust gas grows with the increase of air preheated temperature,which indicates that decreasing air preheated temperature under the premise of ensuring normal operation of coke ovens is another effective way to reducing NOx in coke oven combustion chambers. In addition,the NOx concentrations in all cases maintain between 450 mg·m-3 to 650 mg·m-3,which are in agreement with experimental data in literature [29] and lower than the current emission standard in China.
|
| Fig.10 The NOx concentrations in exhaust gas of case 2~6 |
This paper is targeted on the research of distribution of NOx and the effects of exhaust gas recirculation process and air preheated temperature on the formation of NOx in a coke oven combustion chamber by establishing models of k-ε,EDC,DO and thermal-NO to describe the flow,combustion,heat transfer and NOx formation in the combustion chamber. The main conclusions including:
1) The distribution of NOx in the upward vertical flue and the downward vertical flue is different. Due to the influence of temperature and O2 concentration,the distribution of NOx in the upward vertical flue is uneven,while the distribution of NOx in the downward vertical flue is uniform,which is only controlled by O2 concentration.
2) The exhaust gas recirculation process,which decreases the temperature of gas mixtures and the O2 concentration near combustion zone,is an effective way to reduce NOx concentration in exhaust gas.
3) Higher air preheated temperature,causing higher temperature in the combustion chamber,leads to a growth in NOx emission. Therefore,decreasing air preheated temperature on condition of ensuring normal operation of coke ovens is another effective way to reduce NOx emission.
| [1] | Hao Dandan. Numerical simulation of carbonization process and macro-scale operations technology for coke oven[D]. Tianjin: Tianjin University, 2012(in Chinese) |
| [2] | Zhang Xinxin, Zhang Anqiang, Feng Yanhui, et al. Energy consumption analysis and technologies of waste heat utilization for coke oven[J]. Iron and Steel, 2012, 47(8): 1-12(in Chinese) |
| [3] | Zhong Yingfei. Formation mechanism and control of NOx during coke oven heating and combustion[J]. Fuel & Chemical Processes, 2009, 40(6): 5-12 (in Chinese) |
| [4] | Rowland F S. Stratospheric ozone depletion[J]. Philosophical Transactions of The Royal Society B-Biological Science, 2006, 361(1469): 769-790 |
| [5] | Jin Y M, Veiga M C, Kennes C. Bioprocesses for the removal of nitrogen oxides from polluted air[J]. Journal of Chemical Technology and Biotechnology, 2005, 80(5): 483-494 |
| [6] | Naeher L P, Brauer M, Lipsett M, et al. Woodsmoke health effects: A review[J]. Inhalation Toxicology, 2007, 19(1): 67-106 |
| [7] | Ding Weixia. Study of numerical simulation of staged combustion reducing NOx emission of 300 MW boiler[D]. Beijing: North China Electric Power University, 2006 |
| [8] | Fu G. NOx formation mechanism and control technology in the process of coal combustion[J]. Energy Environmental Protection, 2005, 19(3): 1-8 (in Chinese) |
| [9] | Jin J, Fan J, Sha Y, et al. Experimental research and numerical simulation on NOx release characteristics along the boiler during pulverized coal combustion[J]. Energy & Fuel, 2010, 24: 940-944 |
| [10] | Choi C R, Kim C N. Numerical investigation on the flow, combustion and NOx emission characteristics in a 500 MW tangentially fired pulverized-coal boiler[J]. Fuel, 2009, 88: 1 720-1 731 |
| [11] | Liu B, Yang X, Song W, et al. Process simulation of formation and emission of NO and N2O during coal decoupling combustion in a circulating fluidized bed combustor using Aspen Plus[J]. Chemical Engineering Science, 2012, 71: 375-391 |
| [12] | Cao H, Sun S, Liu Y, et al. Computational fluid dynamics modeling of NOx reduction mechanism in oxy-fuel combustion[J]. Energy & Fuel, 2010, 24: 131-135 |
| [13] | Zhou H, Mo G, Si D, et al. Numerical simulation of the NOx emission in a 1 000 MW tangentially fired pulverized-coal boiler: Influence of the multi-group arrangement of the separated over fire air[J]. Energy & Fuel, 2011, 25: 2 004-2 012 |
| [14] | Li Xiaolei, Xiong Yangheng, Mo Chongjun. Numerical simulation on air-staged low NOx combustion system in a 1 000 MW-class ultra-supercritical boiler[C]//IEEE. Asia-Pacific Power and Energy Engineering Conference. Wuhan, China: 2012(in Chinese) |
| [15] | Li Z, Wei F, Jin Y. Numerical simulation of pulverized coal combustion and NOx formation[J]. Chemical Engineering Science, 2003, 58: 5 161-5 171 |
| [16] | Hao D, Liu W, Dang L, et al. Numerical simulation of a coke oven[J]. Advanced Materials Research, 2012: 586-589 |
| [17] | Wang Fujun. Computational fluid dynamics: CFD principles and applications[M]. Beijing: Tsinghua University Press, 2004 (in Chinese) |
| [18] | Hellberg P, Jonsson T L I, Jonsson P G. Mathematical modeling of the injection of coke oven gas into a blast furnace tuyere[J]. Scandinavian Journal of Metallurgy, 2005, 34: 269-275 |
| [19] | Liu Daifei, Ding Fengqi, Zhang Hongliang, et al. Numerical simulation of high temperature air combustion in aluminum hydroxide gas suspension calcinations[J]. Transactions of Nonferrous Metals Society of China, 2009, 19: 259-266(in Chinese) |
| [20] | Magnussen B F. On the structure of turbulence and a generalized eddy dissipation concept for chemical reaction in turbulent flow[C]//Proceedings of the 19th AIAA Aerospace Science Meeting, St. Louis, United States:1981 |
| [21] | Cui K, Liu B, Zhang H, et al. Modeling of pulverized coal combustion in turbulent flow with the consideration of intermediate reactions of volatile matter[J]. Energy & Fuels, 2013, 27: 2 246-2 254 |
| [22] | Yao Zhaozhang, Zheng Mingdong. Coking plant science[M]. Beijing: Metallurgical Industry Press, 2008 (in Chinese) |
| [23] | Fiveland W A. Three-Dimensional radiative heat-transfer solutions by the discrete-ordinates method[J]. Journal of Thermophysics and Heat Transfer, 1988, 2(4): 309-316 |
| [24] | Stamnes K, Tsay S C, Wiscombe W, et al. Numerically stable algorithm for discrete-ordinate-method radiative transfer in multiple scattering and emitting layered media[J]. Applied Optics, 1988, 27(12): 2 052-2 059 |
| [25] | Backreedy R I, Jones J M, Ma L, et al. Prediction of unburned carbon and NOx in a tangentially fired power station using single coals and blends[J]. Fuel, 2005, 84: 2 196-2 203 |
| [26] | Ma L, Gharebaghi M, Porter R, et al. Modelling methods for co-fired pulverised fuel furnaces[J]. Fuel, 2009, 88(12): 2 448-2 454 |
| [27] | Huang Zhonglang. Research on engineering application of membrane method oxygen-enrichment local supporting combustion technigue on decreasing NOx emission of pulverized coal boiler[D]. Changsha: Central South University, 2007 (in Chinese) |
| [28] | Ou Jianping. Study on application of HTAC in Metallurgy and its optimization with numerical simulation[D]. Changsha: Central South University, 2004 (in Chinese) |
| [29] | Zhong Yingfei. Controlling pollutant discharge quantity and improving the present situation of cokemaking environment[J]. Fuel & Chemical Processes, 2000, 31(6): 277-283(in Chinese) |
 2016, Vol. 33
2016, Vol. 33