2. 南京工业大学材料化学工程国家重点实验室, 南京 210009;
3. 淮阴工学院生命科学与化学工程学院, 江苏淮安 223003
2. State Key Laboratory of Materials-Oriented Chemical Engineering, Nanjing University of Technology, Nanjing 210009, China;
3. Life Science and Chemical Engineering School, Huaiyin Institute of Technology, Huaian 223003, China
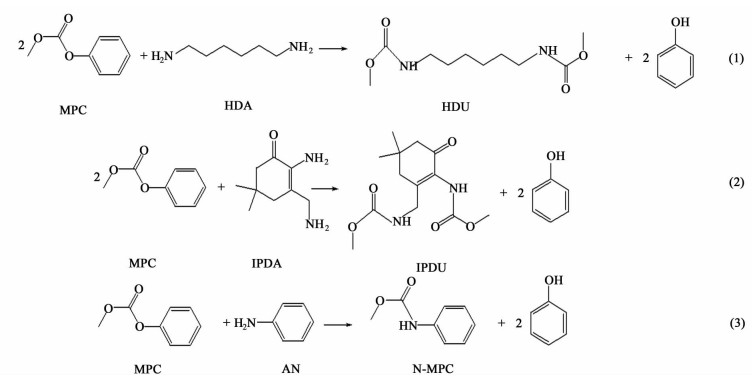
异氰酸酯是聚氨酯工业的主要原料之一,其工业生产方法以光气法为主。该工艺存在以下缺点:原料光气剧毒,副产氯化氢,环境危害严重[1]。目前研究较多的是以碳酸二甲酯(DMC)与胺经甲氧羰基化反应制得氨基甲酸酯[2, 3],氨基甲酸酯再经过热裂解生成相应的异氰酸酯[4]。该路线可有效避免光气所带来的环境危害,副产甲醇可回收制备原料DMC,实现原子经济反应。与DMC相比,甲基苯基碳酸酯(MPC)与胺进行甲氧羰基化反应具有原料转化率高、反应条件温和、副反应少、产物选择性高等优点,是一条颇具工业前景的工艺路线[5, 6]。选取了1,6-六亚甲基己二胺(HDA)、异佛尔酮二胺(IPDA)和苯胺(AN)与MPC甲氧羰基化反应的三个反应体系,采用基团贡献法进行了热力学计算和热力学分析,以期对试验研究以及可能的工业化生产提供理论依据。反应方程式如(1)~(3)所示。
在热力学计算中,除甲醇和苯酚的热力学数据可直接由文献获得外,其它物质理想气体的标准摩尔生成焓变ΔfHmΘ(g)、绝对熵SmΘ(g)和热容CpΘ等数据采用Benson法[7]和Joback[8] 法估算。
1.1 298.15 K时理想气体的标准摩尔生成热ΔfHmΘ(g)和绝对熵SmΘ(g)的计算各物质理想气体的标准摩尔生成焓变ΔfHmΘ(g)和标准SmΘ(g)的计算采用Benson基团贡献法,ΔfHmΘ(g)和SmΘ(g)的计算由(4)式和(5)式给出[7]:
| $ {\Delta _f}H_{g,298}^\Theta = \sum {{n_j}\Delta {H_j}} $ | (4) |
| $ S_m^\Theta \left( g \right) = \sum {{n_j}\Delta {S_j} - R\ln \sigma + R\ln \eta } $ | (5) |
ΔfHmΘ(g)和SmΘ(g)的各基团贡献值如表 1所示。
| 基团 | n i(HDA) | n i(HDU) | n i(IPDA) | n i(IPDU) | n i(苯胺) | n i(N-MPC) | n i(苯酚) | n i(MPC) | Δ f H Θ g/(kJ·mol -1) | Δ S Θ g/(J·mol -1·K -1) |
| 其中C—(O)(H)3C—(Cd)(H)3;C—(Cd)(N)(H)2C—(N)(C)(H)2;CO—(N)(O)C—(N)(C)(H)2;CO—(N)(O)CO—(N)(C);N—(Cd)(H)2N—(C)(H)2;N—(Cd)(CO)(H)N—(CO)(C)(H);CO—(O)2CO—(O)(C);其中N—(CB)(CO)(H) 的ΔSΘm值用N—(CO)(C)(H)代替;O—(CB)(CO)的ΔSΘm值用O—(CO)(C)代替。 | ||||||||||
| C—(C) 2(H) 2 | 4 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | -20.72 | 39.44 |
| C d—(H) 2 | 0 | 0 | 3 | 3 | 0 | 0 | 0 | 0 | 26.21 | 115.60 |
| C—(C d)(N)(H) 2 | 2 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | -27.60 | 41.0 |
| N—(C)(H) 2 | 2 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 20.10 | 124.4 |
| N—(CO)(C)(H) | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | -18.40 | 16.3 |
| CO—(N)(O) | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 0 | -137.30 | 67.80 |
| O—(CO)(C) | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 1 | -185.48 | 35.13 |
| C—(O)(H) 3 | 0 | 2 | 3 | 3 | 0 | 1 | 0 | 1 | -42.20 | 127.32 |
| C d—(C) 2 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 43.29 | -53.17 |
| C d—(N)(H) | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | -21.80 | 49.0 |
| N—(C d)(CO)(H) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | -18.40 | 16.3 |
| C B—(H) | 0 | 0 | 0 | 0 | 5 | 5 | 5 | 5 | 13.82 | 48.72 |
| C B—(N) | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | -2.1 | -40.6 |
| N—(C B)(CO)(H) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1.7 | 16.3 |
| O—(C B)(CO) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | -136.07 | 35.13 |
| CO—(O) 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | -125.50 | 20.01 |
| C—(C) 4 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 2.09 | -146.96 |
| O—CB—H | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | -158.68 | 121.84 |
| CB—O | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | -3.77 | -42.71 |
要计算不同温度下各组分的生成热,需要不同温度下的热容Cp。Benson法[8]计算热容精度较好,但Benson法给出基团的热容数据较少,温度间隔太大。因此,采用精度较好,处理物质种类较多的Joback法[8, 9]计算各物质热容。
Joback法计算热容公式为:
| $ \begin{array}{l} {C_p}\left( T \right) = \left( {\sum {\Delta a - 37.93} } \right) + \\ \left( {\sum {\Delta b + 0.21} } \right)T + \left( {\sum {\Delta c - 3.91 \times {{10}^{ - 4}}} } \right){T^2} + \\ \left( {\sum {\Delta d + 2.06 \times {{10}^{ - 7}}} } \right){T^3} \end{array} $ | (6) |
Joback法各基团对热容的贡献值如表 3所示,各物质所含基团种类及个数如表 4所示。利用表 3和表 4中数据及上述公式,计算得到各物质热容计算式如表 2所列。
| 物质 | Δ f H Θ g298/(kJ·mol -1) | Δ S Θ m/(J·mol -1·K -1) | C Θ p /(J·mol -1·K -1) |
| HDA | -97.88 | 482.8 | 10.42+0.6976T+8.33×10 -4T 2+8.22×10 -7T 3 |
| HDU | -904.84 | 708.8 | 42.20+0.997T+8.1620×10 -4T 2+1.4600×10 -8T 3 |
| IPDA | -193.78 | 517.76 | -146.93+1.731T-1.457×10 -4T 2+7.33×10 -7T 3 |
| IPDU | -1 000.90 | 743.79 | -115.15+2.030T-1.4378×10 -3T 2+6.114×10 -7T 3 |
| 苯胺 | 87.10 | 321.63 | -29.98+0.5568T-8.22×10 -5T 2+9.67×10 -8T 3 |
| N-MPC | -316.47 | 440.42 | -13.19+0.7063T-1.0160×10 -4T 2+6.29×10 -8T 3 |
| MPC | -424.01 | 403.58 | 20.07+0.5305T+9.31×10 -5T 2-9.14×10 -9T 3 |
| 苯酚 | -93.35 | 316.97 | -54.07+0.709T-3.622×10 -4T 2+2.4370×10 -7T 3 |
| 基团 | Δa/(J·mol-1·K-1) | Δb/(J·mol-1·K-2) | Δc/(J·mol-1·K-3) | Δd/(J·mol-1·K-4) | Δoi/(kJ·mol-1) | Δli/(kJ·mol-1) | ΔTb,i/K | ΔTc,i/K |
| CH3— | 1.95E-1 | -8.08E-3 | 1.53E-4 | -9.67E-8 | -17.520 | 70.037 | 23.58 | 0.0141 |
| —O—(非环) | 2.55E+1 | -6.32E-2 | 1.11E-4 | -5.48E-8 | 158.991 | 115.484 | 22.42 | 0.0168 |
| >CO(非环) | 6.45 | 7.70E-2 | -3.57E-5 | 2.86E-9 | 644.875 | -428.108 | 76.75 | 0.0380 |
| —COO—(酯) | 2.45E+1 | 4.02E-2 | 4.02E-5 | -4.52E-8 | 363.706 | 1 315.350 | 81.10 | 0.0481 |
| —NH2 | 2.69E+1 | -4.12E-2 | 1.64E-4 | -9.76E-8 | 286.164 | 410.002 | 73.23 | 0.0243 |
| —CH2— | -9.09E-1 | 9.50E-2 | -5.41E-5 | 1.19E-8 | 190.833 | -27.338 | 22.88 | 0.0189 |
| —NH— | -1.21 | 7.62E-2 | -4.86E-5 | 1.05E-8 | 113.081 | 1 155.660 | 50.17 | 0.0295 |
| —CH2—(环) | -6.03 | 8.54E-2 | -8.00E-6 | -1.80E-8 | 66.607 | 56.308 | 27.15 | 0.0100 |
| —CH<(环) | -2.05E-1 | 1.62E-1 | -1.60E-4 | 6.24E-8 | 318.838 | -309.575 | 21.78 | 0.0122 |
| >C<(环) | -9.09E+1 | 5.57E-1 | -9.00E-4 | 4.69E-7 | 448.250 | -995.223 | 21.32 | 0.0042 |
| CH—(环) | -2.14 | 5.74E-2 | -1.64E-6 | 1.59E-8 | 176.209 | -90.751 | 26.73 | 0.0082 |
| >C(环) | -8.25 | 1.01E-1 | -1.42E-4 | 6.78E-8 | 76.720 | 139.472 | 31.01 | 0.0143 |
| —OH(酚) | -2.81 | 1.11E-1 | -1.16E-4 | 4.94E-8 | 902.248 | 1 431.290 | 76.34 | 0.0240 |
| 基团 | HDA | HDU | IPDA | IPDU | 苯胺 | N-MPC | 苯酚 | MPC |
| CH3— | 0 | 2 | 3 | 5 | 0 | 1 | 0 | 1 |
| —O—(非环) | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 1 |
| >CO(非环) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| —COO—(酯) | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 1 |
| —NH2 | 2 | 0 | 2 | 2 | 1 | 0 | 0 | 0 |
| —CH2— | 6 | 6 | 1 | 0 | 0 | 0 | 0 | 0 |
| —NH— | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| —CH2—(环) | 0 | 0 | 3 | 3 | 0 | 0 | 0 | 0 |
| —CH<(环) | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| >C<(环) | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 |
| CH—(环) | 0 | 0 | 0 | 0 | 5 | 5 | 5 | 5 |
| >C(环) | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
| —OH(酚) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
本体系所讨论的是液相反应,要计算各组分液态生成热,需要各组分的汽化潜热。
首先利用马沛生提出的基团贡献法[7],计算正常沸点下的蒸发热,各基团贡献值如表 3所示,计算公式为:
| $ {\Delta _v}H_b^2 = 152.8834 + \sum {{n_i}\left( {\Delta _i^0 + {X_i}\Delta _i^1} \right)} $ | (7) |
然后利用Watson法[7]
| $ \Delta {H_v} = {\Delta _v}{H_b} \times {\left[{\left( {1 - {T_r}} \right)/\left( {1 - {T_{br}}} \right)} \right]^n} $ | (8) |
计算不同温度下汽化潜热ΔHv,计算中需要的正常沸点温度Tb及临界温度Tc均利用Joback基团加合法计算,各基团贡献值列于表 3。ΔHv计算式中需要的各物质n由式(9)得到:
| $ n = {\left( {0.00264\frac{{{\Delta _v}{H_b}}}{{R{T_b}}} + 0.8794} \right)^{10}} $ | (9) |
由此各物质沸点下蒸发焓、正常沸点温度、临界温度和各温度下蒸发热计算公式如表 5所列。
| 物质 | ΔvHb/(kJ·mol-1) | Tb/K | Tc/K | ΔHv/(kJ·mol-1) |
| HDA | 44.25 | 481.74 | 674.62 | ΔHv=70.91(1-T/674.62)0.3767 |
| HDU | 54.44 | 644.98 | 824.44 | ΔHv=97.07(1-T/824.44)0.3767 |
| IPDA | 45.87 | 584.15 | 819.22 | ΔHv=73.38(1-T/742.54)0.3799 |
| IPDU | 50.77 | 747.39 | 956.08 | ΔHv=90.32(1-T/1015.71)0.3610 |
| 苯胺 | 36.03 | 457.00 | 689.80 | ΔHv=49.88(1-T/698.9)0.3606 |
| N-MPC | 44.41 | 510.12 | 725.11 | ΔHv=61.98(1-T/746.82)0.3649 |
| MPC | 43.62 | 500.44 | 715.50 | ΔHv=66.80(1-T/736.45)0.3741 |
| 苯酚 | 45.39 | 439.20 | 692.35 | ΔHv=65.75(1-T/702.55)0.3893 |
各组分生成热和绝对熵的计算公式:
| $ \begin{array}{l} {\Delta _f}{H_m}\left( {l,T} \right) = {\Delta _f}{H_m}\left( {g,T} \right) - \Delta {H_v}\left( T \right) = \\ {\Delta _f}H_{g,298}^\Theta + \int_{298}^T {{C_{p,g}}dT - \Delta {H_v}\left( T \right)} \end{array} $ | (10) |
| $ {S_m}\left( {l,T} \right) = S_m^\Theta + \int_{298}^T {\frac{{{C_p}}}{T}dT - \frac{{\Delta {H_v}\left( T \right)}}{T}} $ | (11) |
计算结果见表 6。
| 物质 | Δ f H m (l,T)/(kJ·mol -1) | S m (l,T)/(J·mol -1·K -1) | ||||||
| 298 K | 348 K | 358 K | 368 K | 298 K | 348 K | 358 K | 368 K | |
| HDA | -155.11 | -135.78 | -131.50 | -129.10 | 290.75 | 377.49 | 393.94 | 402.65 |
| HDU | -986.37 | -960.89 | -955.31 | -952.45 | 435.75 | 533.19 | 575.25 | 586.18 |
| IPDA | -254.70 | -238.14 | -234.51 | -232.66 | 313.33 | 393.43 | 408.42 | 415.78 |
| IPDU | -1 077.75 | -1 055.05 | -1 050.11 | -1 047.60 | 485.90 | 529.20 | 536.46 | 539.93 |
| 苯胺 | 43.46 | 52.83 | 54.88 | 55.96 | 175.20 | 224.75 | 233.89 | 238.44 |
| N-MPC | -373.71 | -360.81 | -358.00 | -356.78 | 293.98 | 353.12 | 364.12 | 369.60 |
| MPC | -480.03 | -467.33 | -464.57 | -463.17 | 215.60 | 281.35 | 293.42 | 299.33 |
| 苯酚 | -265.21 | -254.90 | -252.67 | -251.53 | 128.30 | 186.86 | 197.52 | 203.69 |
用下列各式计算各反应的反应焓差ΔrH、熵变ΔrS、吉布斯自由能变化ΔrG、平衡常数Kp:
| $ \sum {{{\left( {{n_i}{\Delta _f}{H_{m,L}}} \right)}_{反应物}}} $ | (12) |
| $ \begin{array}{l} {\Delta _f}{S_m} = \sum {{{\left( {{n_i}{\Delta _f}{S_{m,L}}} \right)}_{产物}} - } \\ \;\;\;\;\sum {{{\left( {{n_i}{\Delta _f}{S_{m,L}}} \right)}_{反应物}}} \end{array} $ | (13) |
| $ \Delta G = {\Delta _r}H - T{\Delta _r}S $ | (14) |
| $ {K_n} = \exp \left[{ - {\Delta _r}G/\left( {RT} \right)} \right] $ | (15) |
反应体系在不同温度时的焓差、吉布斯自由能变化和平衡常数的计算结果如表 7所示。
| 温度/K | 反应1 | 反应2 | 反应3 | ||||||
| Δ r H/(kJ·mol -1) | Δ r G/(kJ·mol -1) | K p | Δ r H/(kJ·mol -1) | Δ r G/(kJ·mol -1) | K p | Δ r H/(kJ·mol -1) | Δ r G/(kJ·mol -1) | K p | |
| 298 | -170.34 | -161.52 | 2.055E+28 | -162.13 | -161.53 | 2.060E+28 | -86.71 | 373.01 | 4.120E-66 |
| 348 | -168.81 | -164.19 | 4.421E+24 | -160.61 | -142.59 | 2.132E+21 | -85.49 | 389.22 | 3.768E-59 |
| 358 | -168.51 | -164.76 | 1.096E+24 | -160.30 | -137.47 | 1.146E+20 | -85.23 | 392.91 | 4.674E-58 |
| 368 | -168.12 | -165.30 | 2.907E+23 | -159.90 | -132.59 | 6.612E+18 | -84.94 | 396.33 | 5.522E-57 |
由表 7可见:除反应(3),反应(1)和(2)的吉布斯自由能ΔrG均为负值,在所计算的温度、压力范围内反应为自发过程,且Kp都很大,反应极易进行;反应(1)和(2)的ΔrH均为负值,说明反应均为放热反应,且Kp随温度的升高而减小,但变化不大,说明MPC可以在温度温和的条件下与胺进行甲氧羰基化反应制备氨基甲酸甲酯;反应(2)的ΔrG最小和Kp最大,说明MPC与链状脂肪族胺的甲氧羰基化反应最易进行,其次是环状脂肪族胺,与芳香族胺最不易进行甲氧羰基化反应。
3 结论在缺少热力学数据的情况下,用Benson法、Joback法等多种估算法,计算了MPC与3种胺进行甲氧羰基化反应体系中各组份的热力学数据,并对三种反应体系进行了热力学分析和比较。计算结果表明,MPC与脂肪族二胺合成氨基甲酸甲酯的反应具有较小的ΔrG和较大的Kp,该工艺具有一定的工业应用价值。
符号说明:CpΘ—气体热容,J·mol-1·K-1;
Η和σ—分别为物质的光学异构体数和物质的对称数;
ni—基团个数;
SmΘ(g)和SmΘ(l)—分别为气体和液体的标准熵,J·mol-1·K-1;
Tb和Tbr—分别为正常沸点温度和正常沸点的对比温度,K;
Tc和Tr—分别为临界温度和对比温度,K;
Δa—热容计算的基团贡献值,J·mol-1·K-1;
Δb—热容计算的基团贡献值,J·mol-1·K-2;
Δc—热容计算的基力贡献值,J·mol-1·K-3;
ΔrG和ΔrH—分别为吉布斯自由能变化和反应焓变,kJ·mol-1;
ΔvHb和ΔHv—分别为正常沸点和不同温度下汽化潜热,kJ·mol-1;
ΔfHmΘ(g)和ΔfHmΘ(l)—分别气体和液体标准摩尔生成焓变,kJ·mol-1;
ΔrS—反应的熵变,J·mol-1·K-1;
Δ0i和Δ1i—蒸发热的基团贡献值,kJ·mol-1。
| [1] | 刘玉海,赵辉,李国平,等. 异氰酸酯[M]. 北京:化学工业出版社,2004 Liu Yuhai, Zhao Hui, Li Guoping, et al. Isocyanates[M]. Beijing: Chemical Industry Press, 2004 |
| [2] | Fu Z, Ono Y. Synthesis of methyl N-phenyl carbamate by methoxycarbonylation of aniline with dimethyl carbonate using Pb compounds as catalysts[J]. J Mol Catal, 1994, 3(91): 399-405 |
| [3] | Vauthey I, Valot F, Gozzi C, et al. An environmentally benign access to carbamates and ureas[J]. Tetrahedron Lett, 2000, 41(33): 6 347-6 350 |
| [4] | 赵新强,王延吉,李芳,等. 用碳酸二甲酯代替光气合成甲苯二异氰酸酯[J]. 精细化工,2000,17(10): 615-617 Zhao Xinqiang, Wang Yanji, Li Fang, et al. Synthesis of tolylene diisocyanate with dimethyl carbonate instead of phosgene II. Cleavage of tolylene dicarbamate[J]. Fine Chemicals, 2000, 17(10): 615-617 (in Chinese) |
| [5] | Tsutomu Y, Masaaki S, Fumiaki H, et al. Highly selective methoxycarbonylation of aliphatic diamines with methyl phenyl carbonate to the corresponding methyl N-alkyl dicarbamates[J]. Applied Catalysis A: General, 2005, 289(2): 174-178 |
| [6] | 邱志燕,杨勇,崔咪芬,等. 无溶剂法合成1,6-六亚甲基二氨基甲酸甲酯[J]. 精细石油化工,2010,27(5): 25-27 Qiu Zhiyan, Yang Yong, Cui Mifen, et al. Solvent-Free synthesis of dimethylhexane-1,6-dicarbamate[J]. Speciality Petrochemicals, 2010, 27(5): 25-27 (in Chinese) |
| [7] | 马沛生. 化工数据[M]. 北京:中国石化出版社,2003 Ma Peisheng. Chemical engineering data[M]. Beijing: China Petrochemical Press, 2003 (in Chinese) |
| [8] | 王福安,江登高. 化工数据导引[M]. 北京:化学工业出版社,1995 Wang Fuan, Jiang Denggao. Introduction of chemical engineering data[M]. Beijing: Chemical Industry Press, 1995 (in Chinese) |
| [9] | 李华昌,符宾. 实用化学手册[M]. 北京:化学工业出版社,2006 Li Huachang, Fu Bin. Manual of practical chemistry[M]. Beijing: Chemical Industry Press, 2006 (in Chinese) |
 2016, Vol. 33
2016, Vol. 33