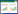粉煤灰又称飞灰,其粒径一般在1~100 μm之间,是在燃煤过程中从烟气中捕集下来的细小固体颗粒物,为一种重要的工业固体废弃物。其主要成分是SiO2、Al2O3和Fe2O3,同时含有少量的CaO、MgO、K2O和SO3等氧化物以及微量的Cr、Hg和Pb等重金属元素。我国粉煤灰产量巨大,堆放处理不仅会侵占土地资源,造成大面积土地资源的浪费,释放到空气中还会造成大气污染,其中的重金属流入水体或渗入土壤中,也会破坏水土结构及其稳定性,甚至通过生物富集作用威胁人体健康,粉煤灰的处置问题亟待解决。近年来,粉煤灰的综合利用水平逐渐提高,广泛应用在污水处理、废气处理、土壤改良、金属及金属氧化物的提取、建筑材料、陶瓷制造、填充材料等领域[1-8],并表现出优良的性能,因此粉煤灰的利用在资源利用与环境保护方面均具有现实意义。
沸石是由硅氧四面体和铝氧四面体通过“氧桥”以共顶角方式连接而成的一种含水的碱或碱土金属铝硅酸盐矿物[9],具有特殊的三维格架,沸石格架中包含各种均匀分布的空穴和通道。粉煤灰中通常含有大量的SiO2和Al2O3,与沸石的主要组分相似,因此可用于沸石的合成。尽管粉煤灰中含有多种重金属元素,但在合成沸石的过程中,大部分金属可以被固化稳定化,在不同pH值条件下均没有明显的浸出,因此利用粉煤灰生产的沸石被认为是安全的[10]。粉煤灰合成沸石拓宽了粉煤灰在高附加值领域的应用,较大的比表面积、良好的吸附性、阳离子交换性、催化性使其广泛应用于水处理中[11],是一种新型的、性能优异的水处理剂。但粉煤灰沸石的亲水性、单一的孔道结构及存在杂质等问题均限制了其在实际中的应用,因此本论文对粉煤灰沸石的改性方法及其在水处理中的应用进行了综述,并对今后的发展方向进行了展望。
1 粉煤灰合成沸石简介粉煤灰沸石的合成方法包括常规水热合成法(一步法、两步法)[12-16]、碱熔融水热法[17, 18]、微波辅助法[19-21]、超临界水热法[22, 23]等,其特点如表 1所示。由于粉煤灰来源和燃烧方式的不同,其化学成分差别较大,故选用的合成方法与相应产物也存在差异[14]。以粉煤灰为原料合成沸石的常见类型、典型合成方法与制备工艺如表 2所示。
| 合成方法 | 优点 | 缺点 |
| 一步水热合成法 | 操作简单、反应周期短 | 纯度低、易产生副产物 |
| 两步水热合成法 | 转化率高、纯度高 | 操作复杂,成本高 |
| 碱熔融水热法 | 转化率高、纯度高 | 活化时间长、能耗较高,操作步骤繁琐 |
| 微波辅助法 | 反应速率快,合成时间短,能耗低 | 转化率低、副产物多 |
| 超临界水热法 | 反应速率快、结晶度高 | 成本高 |
| 类型 | 骨架结构 | 硅铝比 | 主要特征 | 典型合成方法 | 制备工艺 | 应用领域 |
| X型(NaX、13X) | FAU | 1.1~1.5 | 孔径、比表面积大 | 碱熔融水热法 | 将NaOH与粉煤灰混合,高温熔融处理,与水混合陈化,水热晶化,过滤、洗涤、干燥[32, 33] | 废水废气吸附、VOCs催化氧化、催化剂载体、酯交换反应催化剂 |
| A型(4A、NaA) | LTA | 1 | Al离子含量高,离子交换性能强 | 两步水热法 | 将粉煤灰与NaOH溶液混合加热,过滤,向滤液中添加NaAlO2,搅拌,进行水热反应,过滤、洗涤、干燥[34, 35] | 废水处理、气体吸附 |
| P型(NaP1) | GIS | 5∶3 | 具有2种不同大小孔径,离子交换性能、吸附性能良好 | 水热法 | 将粉煤灰与NaOH溶液混合后进行水热处理,过滤、洗涤、干燥[36] | 废水净化 |
| ZSM-5 | MFI | 比表面积大、表面酸性强 | 水热法 | 将粉煤灰、NaOH、四丙基氢氧化铵、正硅酸乙酯与水搅拌后,进行水热晶化反应[37] | VOCs催化氧化、废水处理 |
研究表明,采用粉煤灰合成的沸石是一种较有前景的低成本水处理材料。Cardoso等[24]对比了粉煤灰沸石与商业沸石处理酸性矿山废水的去除效率,虽然粉煤灰沸石的阳离子交换容量仅有商业沸石的一半,但在相同条件下,却可以达到相似的处理效果,且可观察到对Mn2+和Zn2+的去除率高于商业沸石。Sivalingam等[25]利用改良水热法合成了X型微孔粉煤灰沸石,其对结晶紫的最高去除率为99.62%,高于粉煤灰(82.42%)和商用沸石(96.23%)。Attari等[26]研究发现,在相同的实验条件下,以粉煤灰为原料合成的LTA沸石对水中Hg的去除率与商用活性炭相当。
2 粉煤灰合成沸石改性方法粉煤灰沸石因其晶体内部的有序通道以及骨架上的可交换阳离子,而具有较高离子交换容量和吸附性能, 对于大部分重金属阳离子具有较好的去除效果。但不同阳离子的吸附和吸附顺序受其离子和水合离子半径的影响,水合半径较大的离子不易进行离子交换,因此其吸附会受到部分抑制[27]。此外,粉煤灰沸石对废水中含氧阴离子吸附量较低,而且沸石的亲水性也限制了其对有机化合物的吸附。因此,为了提高粉煤灰沸石的性能与应用范围,近年来研究者采用了有机改性、无机改性和磁改性等多种方法对粉煤灰沸石进行了改性处理。常见改性方法的机理、主要改性工艺、特点以及应用范围如表 3所示。
| 改性方法 | 改性原理 | 主要改性工艺 | 特点 | 应用 |
| 阳离子表面活性剂改性 | 沸石表面负电荷吸附阳离子表面活性剂,改变沸石表面的亲疏水性或电负性 | 将表面活性剂与粉煤灰沸石剧烈搅拌,过滤、洗涤、干燥并研磨 | 提高了沸石对阴离子与有机污染物的吸附,但改性过程耗时较长 | 脱氮除磷、苯酚、腐殖酸、双酚A等有机污染物的去除 |
| 官能团改性 | 沸石表面负电荷吸附羟基或氨基等官能团,改变沸石表面电负性、增加官能团 | 将壳聚糖完全溶解在有机酸溶液中,得到壳聚糖溶液,加入粉煤灰沸石剧烈搅拌,过滤、洗涤、干燥并研磨 | 增强了沸石吸附阴离子与有机污染物的能力,但改性过程繁琐、耗时长 | 重金属离子、脱氮除磷、腐殖酸等的吸附 |
| 无机金属盐离子改性 | 金属盐离子与粉煤灰沸石中的阳离子进行离子交换,增大孔径,或使沸石表面羟基化 | 将金属盐溶液与粉煤灰沸石混合,进行离子交换,干燥后得到改性产品 | 吸附性能得到很大提升,但其中稀土元素改性成本较高 | 重金属离子、PO43-的去除 |
| 无机酸、碱改性 | 脱除粉煤灰沸石结构中的Al或Si原子或H+取代粉煤灰沸石中的金属阳离子,提高比表面积、孔径 | 将粉煤灰沸浸泡到酸/碱溶液中,水洗至中性、干燥 | 操作简单、方便,吸附性能有所提高,但改性较为耗时,且易破坏沸石晶体结构,易造成二次污染 | 脱氮除磷 |
| 磁改性 | 将磁性材料与粉煤灰沸石结合,使粉煤灰沸石具有磁性,便于回收 | 在合成粉煤灰沸石的过程中加入含Fe试剂一步合成磁改性沸石 | 可重复使用性高 | 部分阳离子污染物的脱除 |
利用表面活性剂对粉煤灰沸石改性时,沸石表面的负电荷可以吸附阳离子表面活性剂,在表面形成活性双分子层(见图 1),使沸石表面具有疏水性,增强了对有机污染物、阴离子的吸附[28, 29]。十六烷基三甲基溴化铵(HDTMA)为粉煤灰沸石改性中最常用的表面活性剂。

|
| 图 1 表面活性剂改性粉煤灰沸石 Fig.1 Surfactant-modified zeolite synthesized from fly ash |
| |
Xie等[30]研究发现,HDTMA改性粉煤灰沸石对可电离性酚类化合物(苯酚、对氯苯酚、双酚A)和不可电离性有机化合物(萘、硝基苯、苯胺)的去除效率提高了几十甚至几千倍。高立祥等[31]经水热法合成了NaP1型粉煤灰沸石(ZFA),经CTAB改性后,对甲基橙的吸附量为59.6 mg·g-1,比未改性的沸石增大了1.4倍。
利用阳离子表面活性剂改性粉煤灰沸石对水中污染物的去除与多种因素有关。Kamble等[38]发现随着HDTMA-Br添加浓度的增大,表面活性剂分子过多地聚集在沸石表面,会阻碍长链烷基化合物尾部对有机物的吸附形成有机分配,从而降低对有机污染物的去除效率。Xie等[39]发现沸石上表面活性剂的负载量主要取决于碳链长度,并随链长的增加而增加,一般情况下,对有机污染物的吸附量也随之增加。随着链长的减小,表面活性剂链的构象趋于无序。研究表明,链长、表面活性剂的覆盖范围以及链的构象都影响有机污染物吸附的因素。Szala等[40]利用HDTMA改性NaP1型粉煤灰沸石,其对对二甲苯、甲苯和苯的去除效率分别为97%、93%和84%,研究发现,不同污染物的吸附效率差异是由它们的物理和化学性质造成的,污染物分子大小、偶极矩和苯环上甲基的存在都会影响沸石对苯系物的去除。通过对不同影响因素的调节,优化实验条件,可使改性沸石更好的用于不同污染物的处理中。
2.1.2 官能团改性天然多聚糖壳聚糖具有活性羟基和氨基,如图 2所示,在改性过程中,壳聚糖中的氨基被阳离子化并被沸石外表面上的负电荷吸引,但是由于壳聚糖的体积较大,因此仅有沸石外表面的负电荷被壳聚糖占据,无机阳离子污染物仍可与内部孔隙中的负电荷接触。壳聚糖改性沸石对染料、重金属、无机酸、有机污染物均有很好的去除效果[41, 42]。Xie等[42]研究了壳聚糖改性粉煤灰沸石(CMZFA)对水中NH4+、PO43-、HA的吸附性能,发现对3种污染物的最大吸附量分别为16.2、4.1和31.6 mg·g-1,相较于未改性沸石,壳聚糖改性极大地提高了对HA的吸附性能。因此,利用壳聚糖改性粉煤灰沸石是一种潜在的、具有一定前景的同步去除水中多种污染物的材料。
有机改性的2种方法均可在保留原沸石阳离子吸附能力的同时,增加对有机污染物、含氧酸阴离子的去除,是一种多效改性方法,在不同污染物去除中具有良好的适应性与协同作用,具有较好的发展前景。但表面活性剂改性受其种类与沸石对其吸附能力的影响。
2.2 无机改性 2.2.1 无机金属盐离子改性金属离子改性主要是通过离子交换原理,利用一些金属盐离子如Na+、Cu2+、Zn2+和Fe3+等替换粉煤灰沸石中的初始合成阳离子,实现离子交换改性,提高沸石的性能。
Tauanov等[43]采用常规水热法合成了粉煤灰沸石,在AgNO3溶液中浸渍12 h实现离子交换改性,后以NaBH4还原部分Ag,形成纳米复合材料(Ag-ZFA),用于水中Hg+的去除。结果表明,Ag-ZFA在24 h达到最大去除率99%,相较于粉煤灰与粉煤灰沸石(去除率分别为29 d,9.7%和14 d,91.3%),Ag-ZFA的吸附能力更强、吸附时间更短。浸出实验表明,Ag-ZFA对Hg+具有良好的存储能力,在强酸和中性条件下的浸出率均小于1%,比CFA与ZFA低4~8倍。Bonetti等[44]选用CaCl2改性X型粉煤灰沸石,改性后的沸石可去除废水中97%的K+与95%的PO43-。Abdellaoui等[45]利用粉煤灰合成了麦钾沸石,用ZrO2+对其进行改性后,比表面积由9.673 m2·g-1增加到了18.572 m2·g-1,在pH≤5时,对H2PO4-的去除率最高,为98.6%。李思洋[46]采用水热离子交换法,制备了Zn-CHA、Cs-CHA、Cu-CHA 3种改性粉煤灰钾型菱沸石。由于改性离子的相对原子质量大于K+,使得其外层电子数增多,对CO2的分子引力增强;另一方面,外层金属离子的变化也会引起离子之间成键的键长、键角的改变,从而引起沸石功能的变化。3种沸石改性后对CO2气体的吸附能力分别提高了19.41%、37.65%、24.12%。
有研究表明,化合价不同的金属离子交换可能会引起沸石骨架的显著改变,甚至呈现完全不同的多孔结构[47],因此利用该方法改性时,应注意金属离子的选择。
稀土元素La也被应用到一些改性粉煤灰沸石的研究中,La3+易与水溶液中的羟基结合,在沸石上形成羟基化表面,改性沸石因此易与阴阳离子结合生成表面配位络合物,增强吸附性能[48]。Wang等[49]采用一锅法合成了镧改性粉煤灰沸石,用于吸附浅水湖泊沉积物中释放的P组分(活性P、还原-可溶性P、NaOH-P、有机P组分),经过28 d的沉积物岩心培养试验,总P和可溶-活性P释放量平均降低了81.1%和86.9%。虽然利用稀土元素改性沸石具有良好的效果,但稀土价格较高,导致生产成本的增加,并不适合大规模生产使用。
2.2.2 无机酸、碱改性酸和碱分别通过脱铝或脱硅过程完成对沸石的改性,增加Bronsted酸和Lewis酸位点,合成高硅铝比或低硅铝比沸石[50-52],使得沸石比表面积、孔径增大,孔道被疏通,提高其吸附和催化性能。酸、碱改性粉煤灰沸石均是通过酸、碱浸渍法制备,最常用的试剂为HCl、H2SO4和NaOH。
适当的酸处理可以去除沸石孔道中的一部分杂质,疏通孔道,沸石组分中的Ca2+、Na+和K+等被可半径较小的H+替换,形成质子交换位点[53],孔容增大,可使一些分子较大的污染物进入沸石组分中从而被吸附脱除。Zhang等[54]利用H2SO4热处理NaP1-ZFA 6 h得到改性产物,探究了不同浓度(0.01、0.1、0.9和1.8 mol·L-1)的H2SO4改性NaP1-ZFA对其脱氮除磷的影响,研究发现,经0.01 mol·L-1 H2SO4处理能显著提高ZFA对氨氮和磷的去除率,而经高浓度的酸处理后,NaP1晶相被破坏,阳离子交换容量降低,对磷酸盐固定能力减弱。
吕海亮等[55]将NaP1型粉煤灰沸石在NaOH与硫酸铝钾溶液中分别浸泡12与36 h进行改性,经改性的粉煤灰沸石增加了活性比表面积与吸附位点,对饮用水中的F-的去除效率从未改性沸石的10.87%提高到了92.27%左右,吸附过程符合Langmuir吸附等温方程。
利用酸、碱改性粉煤灰沸石,可以有效地提高沸石吸附能力,但其浓度控制不当均会导致沸石表面和内部孔隙结构的破坏,且改性过程耗时较长,处理过程中会伴随着废气与废液的产生,易形成二次污染。
2.3 磁改性传统合成沸石多为粉末状,作为重金属离子、氨氮、染料等污染物的吸附剂已得到广泛应用,但在较为复杂的体系中,采用过滤或离心的方法从液相中分离沸石,其过程较为繁琐耗时;而将沸石与磁性材料结合,获得磁性沸石,在外加磁场的作用下即可实现快速分离回收[56, 57]。
Yamaura等[58]发现利用磁选工艺对磁性粉煤灰沸石(MZ)进行分离与离心工艺分离效果相同。实验利用MZ吸附水中活性橙16染料(RO16),将含有该废水的容器放在磁铁上60 min,测定分离清液的吸光度,得到RO16的回收率为90%,又将该清液离心分离20 min,得到了同样的结果,因此,MZ不仅对RO16具有良好的吸附作用,同时具备的磁性能更有利于分离回收。Yang等[59]利用Fe3O4掺杂粉煤灰原位合成了磁稳定性能良好的磁性粉煤灰沸石(MFZ),当Fe3O4负载量为15.41%时,对水中Cu2+吸附量达到最大值。在最适宜条件下,MFZ对Cu2+的最大饱和吸附量为48.99 mg·g-1,与粉煤灰制备的A型沸石(50.45 mg·g-1)相近,略低于工业A型沸石(53.45 mg·g-1),但其更易从水中分离回收,因此是一种优质的吸附材料。
Sugawara等[60]以粉煤灰、NaOH、FeCl3为原料,采用共沉淀法合成了磁性沸石(MZL),对于0.8 mol·L-1的氨氮溶液,最大去除率可达83.4%,并且在流速为0.5 m·s-1、磁场为2 T的条件下,可对99.8%的MZL进行磁分离,大大提高了MZL的重复利用率。
磁改性粉煤灰沸石不仅对污染物具有良好的处理效果,而且简化了传统工艺的分离过程,使其更易从吸附后的溶液中分离,且回收率较高,降低了一定的分离成本,更有利于粉煤灰沸石的应用。
3 粉煤灰沸石在水处理中的应用 3.1 粉煤灰沸石作吸附剂在水处理中的应用沸石都是由硅氧四面体和铝氧四面体组成的,四面体之间通过1个氧原子顶点连接而成,而铝氧四面体本身不能连接,故其间至少需要连接1个硅氧四面体,铝可替换硅氧四面体中的硅,但铝为3价,故需要1个正价离子来平衡电荷,该离子一般为Ca2+、Na+、K+和Mg2+等,而这些离子易和水中阳离子发生交换;同时,粉煤灰沸石的三维硅铝氧格架结构提供了大量的孔穴和通道,使其具有较大的比表面积和吸附能力,在水处理中多用于重金属离子[61-66]、氨氮[67, 68]、有机污染物(染料[69]、苯系物[70]、酚类化合物[71]、多环芳烃[72]、腐殖酸[28]等)及无机含氧酸阴离子[73-76]等的去除。
Angaru等[77]对Na-A型粉煤灰沸石(FZA)进行改性,合成nZVI/Ni@FZA(nZVI为0价铁Fe0),用于水中重金属Cr6+和Cu2+的处理。nZVI/Ni@FZA(154.11 m2·g-1)复合材料由于金属在FZA(46 m2·g-1)上的分散使得比表面积显著增加,从而提供了更多的吸附与反应位点。研究表明,nZVI/Ni@FZA主要通过吸附、还原、离子交换作用去除这2种重金属离子。溶液中的Cr6+和Cu2+首先被吸附在nZVI/Ni@FZA的活性位点上,通过nZVI/Ni将Cr(Ⅵ)/Cu(Ⅱ)还原为Cr(Ⅲ)和Cu0,Ni作为催化剂增强Fe0的还原能力从而促进该反应。表面的氧化铁层(Fe3O4和Fe2O3)也有助于吸附去除过程。此外,由于沸石具有的阳离子交换容量,小部分Cu2+和Cr3+可以被沸石的阳离子交换位点直接去除。最终对Cr6+和Cu2+吸附量为48.31和147.06 mg·g-1,去除效果明显高于nZVI/FZA和FZA。
Liu等[68]通过在粉煤灰沸石合成过程中添加不同质量的nZVI得到改性Fe-Z粉末,Fe的加入替换了沸石骨架中的Al,使得配位的Na+增多,即增加了Fe-Z中的可交换阳离子。当nZVI质量分数为5%时,改性沸石对NH4+的吸附量为50.2 mg·g-1,其去除机理如式(1)所示。
| $ \mathrm{NH}_4^{+}+(x) \mathrm{Fe}-\mathrm{Z}-\mathrm{Na}^{+} \rightarrow(x) \mathrm{Fe}-\mathrm{Z}-\mathrm{NH}_4^{+}+\mathrm{Na}^{+} $ | (1) |
La对水中磷酸盐具有高亲和力,Goscianska等[78]利用LaCl3溶液对粉煤灰沸石进行离子交换改性,在pH值为5~7时,表现出最大的磷酸盐去除能力。在弱酸性条件下,磷酸盐主要存在形式为H2PO42-,H2PO42-和吸附剂表面因质子化而带有的正电荷通过静电引力和离子交换作用吸附到沸石上从而被去除。而在碱性环境下,OH-与PO43-的竞争性增强,La(OH)3抑制了对PO43-的吸附。
Li等[28]合成了高钙粉煤灰沸石(ZFA F)和低钙粉煤灰沸石(ZFA L),2种沸石对腐殖酸(HA)的吸附能力几乎可以忽略不计,经HDTMA改性后,两者对水中HA的吸附量为126.6和31.6 mg·g-1。pH值是影响吸附行为的最重要参数之一。HA在水溶液中表现为具有负电荷的聚电解质,而ZFA表面经过改性后由负电荷转变为正电荷,ZFA F表面负载的HDTMA量高于ZFA L,在低pH值下,带正电的改性沸石表面会增加对带负电的HA的吸引力,所以SMZFA F吸附量更高。另外,ZFA中含有5%~6%的氧化铁,可以通过配体交换机制与HA结合,通过特定的相互作用增加HA的吸附。pH值降低还会导致金属离子浓度增加,尤其是Ca2+,进而提高HA的吸附。SMZFA F是由高钙飞灰制备的,因此其中溶解的Ca2+的平衡浓度比SMZFA L更高且更依赖于pH值,这也是SMZFA F吸附量更高的原因之一。
3.2 粉煤灰沸石作催化剂载体在水处理中的应用沸石骨架上的铝氧四面体所带的酸性羟基是Bronsted酸位点,Lewis酸则是在非沸石基质、沸石间缺陷及非骨架铝中与层间有空余配位阳离子及四面体中3价阳离子配位形成的酸,粉煤灰沸石骨架中酸性位点及无机阳离子的存在使其具有催化性能,沸石较大的比表面积也可用来做催化剂的载体。粉煤灰沸石常用来做生产生物柴油的催化剂[79, 80]、VOCs氧化催化剂[81, 82]等。
在水处理领域,粉煤灰沸石常被用作催化剂载体。黄宇玫等[83]研究了粉煤灰沸石负载CuO催化剂处理酸性大红GR染料废水,在最适宜条件下可去除废水中90.1%的CODcr和99.97%的色度。Park等[84]以粉煤灰为原料合成了Na-X型高纯沸石,并将其用作Pb-Sn双金属催化剂的载体材料,用于去除水中的硝酸盐。粉煤灰沸石载体增强了金属分散性,增加了Pb和Sn纳米粒子的活性金属位,经过优化后的该双金属催化剂几乎可以完全去除地下水中的硝酸盐,N2的选择性为94%。Subbulekshmi等[85]合成了粉煤灰沸石(FAZ),并通过离子交换固定Cu2+,经焙烧形成CuO-FAZ,作为湿法催化降解水中对硝基酚(PNP)和对硝基苯胺(PNA)的催化剂。仅有FAZ和CuO的存在下,PNP的降解率为28%和38%,对PNA的降解率分别为26%和31%。由于FAZ和CuO的协同作用,使其具有更高的氧化能力,从而产生更多的羟基自由基,促进PNP与PNA的降解,最适宜条件下,对污染物降解率为96%和84%。经过4次回收利用之后,其对PNP、PNA的催化效率仍保持在90%和78%。
Hlekelele等[86]研究表明,粉煤灰沸石负载的TiO2与纳米Ag粒子复合物在紫外光和可见光下均具有比TiO2更好的光活性,其中具有纳米Ag粒子的复合材料光活性最佳。这些复合物在紫外光下的优异光活性主要归因于沸石和纳米Ag粒子可以有效的降低电子-空穴复合速率。
4 结语利用富含SiO2和Al2O3的粉煤灰合成沸石,可生产价格低廉、环境友好的污染物处理材料,减轻了粉煤灰堆放及其造成的环境污染问题。粉煤灰沸石通过阳离子表面活性剂、有机官能团、金属盐离子、无机酸碱以及磁性材料的改性,使得沸石的吸附性能大大提高,有望得到更加广泛的应用。但现有粉煤灰合成沸石改性处理同时存在着耗时、改性试剂价格昂贵以及易产生二次污染等问题,在未来的研究工作中应注重改性方法的简化并寻找一些可替代的廉价改性试剂,例如一些天然材料如壳聚糖、磁铁矿等,或继续贯彻“以废治废”理念,发掘一些可利用的废弃材料应用到改性处理中,以此降低生产成本,提高效率。此外,虽然改性粉煤灰沸石能有效去除多种水体污染物,但粉煤灰沸石功能化制备改性及其对水中典型污染物的去除性能和机理还需进一步深入研究。
改性的根本目的是使粉煤灰沸石的处理效率更高、应用范围更广,对于未经处理的废水中往往存在多种不同性质的污染物,经过有机改性的粉煤灰沸石,作为一种多功能材料同时去除多种污染物具有明显的优势,但目前对于机理以及去除过程中是否存在竞争性的探讨较为有限,深刻的认识改性的过程、机理以及同时去除多种污染物的机理有助于更好地对沸石进行设计与制备,值得进一步深入研究。
| [1] |
YAO Z, JI X, SARKER P K, et al. A comprehensive review on the applications of coal fly ash[J]. Earth-Science Reviews, 2015, 141: 105-121. DOI:10.1016/j.earscirev.2014.11.016 |
| [2] |
YAO Z, XIA M, SARKER P K, et al. A review of the alumina recovery from coal fly ash, with a focus in China[J]. Fuel, 2014, 120: 74-85. DOI:10.1016/j.fuel.2013.12.003 |
| [3] |
MUSHTAQ F, ZAHID M, AHMAD BHATTI I, et al. Possible applications of coal fly ash in wastewater treatment[J]. Journal of Environmental Management, 2019, 240: 27-46. |
| [4] |
GOLEWSKI G L. Green concrete composite incorporating fly ash with high strength and fracture toughness[J]. Journal of Cleaner Production, 2018, 172: 218-226. DOI:10.1016/j.jclepro.2017.10.065 |
| [5] |
PARK S, KIM M, LIM Y, et al. Characterization of rare earth elements present in coal ash by sequential extraction[J]. Journal of Hazardous Materials, 2021, 402: 123760. DOI:10.1016/j.jhazmat.2020.123760 |
| [6] |
KIPKEMBOI B, ZHAO T, MIYAZAWA S, et al. Effect of C3S content of clinker on properties of fly ash cement concrete[J]. Construction and Building Materials, 2020, 240: 117840. DOI:10.1016/j.conbuildmat.2019.117840 |
| [7] |
KIM Y, HWANG S, CHOI J, et al. Valorization of fly ash as a harmless flame retardant via carbonation treatment for enhanced fire-proofing performance and mechanical properties of silicone composites[J]. Journal of Hazardous Materials, 2021, 404: 124202. DOI:10.1016/j.jhazmat.2020.124202 |
| [8] |
MALIK A, THAPLIYAL A. Eco-friendly fly ash utilization: Potential for land application[J]. Critical Reviews in Environmental Science and Technology, 2009, 39(4): 333-366. DOI:10.1080/10643380701413690 |
| [9] |
张大洲, 卢文新, 陈风敬, 等. 粉煤灰水热合成沸石分子筛及其应用进展[J]. 化肥设计, 2018, 56(1): 1-4. ZHANG Dazhou, LU Wenxin, CHEN Fengjing, et al. Hydrothermal synthesis of fly ash into zeolite molecular sieves and its application progress[J]. Chemical Fertilizer Design, 2018, 56(1): 1-4. (in Chinese) |
| [10] |
FENG W, WAN Z, DANIELS J, et al. Synthesis of high quality zeolites from coal fly ash: Mobility of hazardous elements and environmental applications[J]. Journal of Cleaner Production, 2018, 202: 390-400. DOI:10.1016/j.jclepro.2018.08.140 |
| [11] |
崔家新, 王连勇, 张坤, 等. 粉煤灰基沸石处理氮磷废水的研究进展[J]. 硅酸盐通报, 2021, 40(8): 2622-2630. CUI Jiaxin, WANG Lianyong, ZHANG Kun, et al. Research progress on treatment of nitrogen and phosphorus wastewater with fly ash-based zeolite[J]. Bulletin of the Chinese Ceramic Society, 2021, 40(8): 2622-2630. DOI:10.16552/j.cnki.issn1001-1625.20210629.002 (in Chinese) |
| [12] |
QUEROL X, UMAÑA J C, PLANA F, et al. Synthesis of zeolites from fly ash at pilot plant scale. Examples of potential applications[J]. Fuel, 2001, 80(6): 857-865. DOI:10.1016/S0016-2361(00)00156-3 |
| [13] |
CHAREONPANICH M, JULLAPHAN O, TANG C. Bench-scale synthesis of zeolite A from subbituminous coal ashes with high crystalline silica content[J]. Journal of Cleaner Production, 2011, 19(1): 58-63. DOI:10.1016/j.jclepro.2010.08.012 |
| [14] |
石德智, 张金露, 张超, 等. 粉煤灰水热法合成沸石的研究进展[J]. 安全与环境学报, 2016, 16(3): 273-279. SHI Dezhi, ZHANG Jinlu, ZHANG Chao, et al. On the research advances in hydrothermal synthesis of zeolite from the coal fly ash[J]. Journal of Safety and Environment, 2016, 16(3): 273-279. DOI:10.13637/j.issn.1009-6094.2016.03.54 (in Chinese) |
| [15] |
TAUANOV Z, SHAH D, INGLEZAKIS V, et al. Hydrothermal synthesis of zeolite production from coal fly ash: A heuristic approach and its optimization for system identification of conversion[J]. Journal of Cleaner Production, 2018, 182: 616-623. DOI:10.1016/j.jclepro.2018.02.047 |
| [16] |
TANAKA H, SAKAI Y, HINO R. Formation of Na-A and-X zeolites from waste solutions in conversion of coal fly ash to zeolites[J]. Materials Research Bulletin, 2002, 37(11): 1873-1884. DOI:10.1016/S0025-5408(02)00861-9 |
| [17] |
吴迪秀, 罗柳, 贾玉娟, 等. 粉煤灰碱熔融-水热法合成A型沸石及吸附性能研究[J]. 硅酸盐通报, 2019, 38(6): 1873-1877. WU Dixiu, LUO Liu, JIA Yujuan, et al. Synthesis of A-zeolite from coal fly ash by alkali fusion-hydrothermal process and its adsorption research[J]. Bulletin of the Chinese Ceramic Society, 2019, 38(6): 1873-1877. DOI:10.16552/j.cnki.issn1001-1625.2019.06.039 (in Chinese) |
| [18] |
JOSEPH I V, TOSHEVA L, DOYLE A M. Simultaneous removal of Cd(Ⅱ), Co(Ⅱ), Cu(Ⅱ), Pb(Ⅱ), and Zn(Ⅱ) ions from aqueous solutions via adsorption on FAU-type zeolites prepared from coal fly ash[J]. Journal of Environmental Chemical Engineering, 2020, 8(4): 103895. DOI:10.1016/j.jece.2020.103895 |
| [19] |
INADA M, TSUJIMOTO H, EGUCHI Y, et al. Microwave-assisted zeolite synthesis from coal fly ash in hydrothermal process[J]. Fuel, 2005, 84(12/13): 1482-1486. |
| [20] |
崔红梅, 柯灵非, 李芳, 等. 微波辅助加热粉煤灰水热合成沸石的最佳条件[J]. 硅酸盐通报, 2012, 31(4): 969-973. CUI Hongmei, KE Lingfei, LI Fang, et al. The better condition for hydrothermal synthesizing zeolite from coal fly ash under the microwave[J]. Bulletin of the Chinese Ceramic Society, 2012, 31(4): 969-973. DOI:10.16552/j.cnki.issn1001-1625.2012.04.047 (in Chinese) |
| [21] |
FUKASAWA T, HORIGOME A, KARISMA A D, et al. Utilization of incineration fly ash from biomass power plants for zeolite synthesis from coal fly ash by microwave hydrothermal treatment[J]. Advanced Powder Technology, 2018, 29(3): 450-456. DOI:10.1016/j.apt.2017.10.022 |
| [22] |
赵宇雄, 王建成, 韩丽娜, 等. 粉煤灰为原料碱熔融-超临界水热合成沸石的研究[J]. 现代化工, 2016, 36(11): 141-145. ZHAO Yuxiong, WANG Jiancheng, HAN Lina, et al. Synthesis of zeolite from fly ash by alkali fusion-supercritical hydrothermal method[J]. Modern Chemical Industry, 2016, 36(11): 141-145. DOI:10.16606/j.cnki.issn0253-4320.2016.11.033 (in Chinese) |
| [23] |
MA L, HAN L, CHEN S, et al. Rapid synthesis of magnetic zeolite materials from fly ash and iron-containing wastes using supercritical water for elemental mercury removal from flue gas[J]. Fuel Processing Technology, 2019, 189: 39-48. DOI:10.1016/j.fuproc.2019.02.021 |
| [24] |
CARDOSO A M, PAPROCKI A, FERRET L S, et al. Synthesis of zeolite Na-P1 under mild conditions using Brazilian coal fly ash and its application in wastewater treatment[J]. Fuel, 2015, 139: 59-67. DOI:10.1016/j.fuel.2014.08.016 |
| [25] |
SIVALINGAM S, SEN S. Optimization of synthesis parameters and characterization of coal fly ash derived microporous zeolite X[J]. Applied Surface Science, 2018, 455: 903-910. DOI:10.1016/j.apsusc.2018.05.222 |
| [26] |
ATTARI M, BUKHARI S S, KAZEMIAN H, et al. A low-cost adsorbent from coal fly ash for mercury removal from industrial wastewater[J]. Journal of Environmental Chemical Engineering, 2017, 5(1): 391-399. DOI:10.1016/j.jece.2016.12.014 |
| [27] |
KUMAR M M, JENA H. Direct single-step synthesis of phase pure zeolite Na-P1, hydroxy sodalite and analcime from coal fly ash and assessment of their Cs+ and Sr2+ removal efficiencies[J]. Microporous and Mesoporous Materials, 2022, 333: 111738. DOI:10.1016/j.micromeso.2022.111738 |
| [28] |
LI C, DONG Y, WU D, et al. Surfactant modified zeolite as adsorbent for removal of humic acid from water[J]. Applied Clay Science, 2011, 52(4): 353-357. DOI:10.1016/j.clay.2011.03.015 |
| [29] |
管擎宇. 粉煤灰合成沸石去除水中有机污染物的研究[D]. 上海: 上海交通大学, 2010 GUAN Qingyu. Study on removal of organic pollutants from water by zeolite synthesized from fly ash[D]. Shanghai: Shanghai Jiao Tong University, 2010 (in Chinese) |
| [30] |
XIE J, MENG W, WU D, et al. Removal of organic pollutants by surfactant modified zeolite: Comparison between ionizable phenolic compounds and non-ionizable organic compounds[J]. Journal of Hazardous Materials, 2012, 231/232: 57-63. DOI:10.1016/j.jhazmat.2012.06.035 |
| [31] |
高立祥, 边祥成, 戴浩, 等. 改性粉煤灰合成沸石对甲基橙的吸附动力学[J]. 环境工程学报, 2015, 9(4): 1709-1714. GAO Lixiang, BIAN Xiangcheng, DAI Hao, et al. Adsorption kinetics of methyl orange on modified zeolite synthesized from fly ash[J]. Chinese Journal of Environmental Engineering, 2015, 9(4): 1709-1714. (in Chinese) |
| [32] |
崔杏雨, 陈树伟, 闫晓亮, 等. 粉煤灰合成Na-X沸石去除废水中镍离子的研究[J]. 燃料化学学报, 2009, 37(6): 752-756. CUI Xingyu, CHEN Shuwei, YAN Xiaoliang, et al. Study on removal of nickel ions from wastewater by synthesis of Na-X zeolite from fly ash[J]. Journal of Fuel Chemistry and Technology, 2009, 37(6): 752-756. (in Chinese) |
| [33] |
MISHRA T, TIWARI S K. Studies on sorption properties of zeolite derived from Indian fly ash[J]. Journal of Hazardous Materials, 2006, 137(1): 299-303. DOI:10.1016/j.jhazmat.2006.02.004 |
| [34] |
HUI K, CHAO C. Pure, single phase, high crystalline, chamfered-edge zeolite 4A synthesized from coal fly ash for use as a builder in detergents[J]. Journal of Hazardous Materials, 2006, 137(1): 401-409. DOI:10.1016/j.jhazmat.2006.02.014 |
| [35] |
DE AQUINO T F, ESTEVAM S T, VIOLA V O, et al. CO2 adsorption capacity of zeolites synthesized from coal fly ashes[J]. Fuel, 2020, 276: 118143. DOI:10.1016/j.fuel.2020.118143 |
| [36] |
汪飞, 吴德意, 何圣兵, 等. 水热法合成NaP1型粉煤灰沸石的性能表征[J]. 材料工程, 2005, 33(8): 47-50. WANG Fei, WU Deyi, HE Shengbing, et al. Property characterization of NaP1 zeolite from coal fly ash by hydrothermal synthesis[J]. Journal of Materials Engineering, 2005, 33(8): 47-50. (in Chinese) |
| [37] |
FENG R, CHEN K, YAN X, et al. Synthesis of ZSM-5 zeolite using coal fly ash as an additive for the methanol to propylene (MTP) reaction[J]. Catalysts, 2019, 9(10): 788. DOI:10.3390/catal9100788 |
| [38] |
KAMBLE S P, MANGRULKAR P A, BANSIWAL A K, et al. Adsorption of phenol and o-chlorophenol on surface altered fly ash based molecular sieves[J]. Chemical Engineering Journal, 2008, 138(1/2/3): 73-83. |
| [39] |
XIE Q, XIE J, WANG Z, et al. Adsorption of organic pollutants by surfactant modified zeolite as controlled by surfactant chain length[J]. Microporous and Mesoporous Materials, 2013, 179: 144-150. DOI:10.1016/j.micromeso.2013.05.027 |
| [40] |
SZALA B, BAJDA T, MATUSIK J, et al. BTX sorption on Na-P1 organo-zeolite as a process controlled by the amount of adsorbed HDTMA[J]. Microporous and Mesoporous Materials, 2015, 202: 115-123. DOI:10.1016/j.micromeso.2014.09.033 |
| [41] |
KOŁODYŃSKA D, HAŁAS P, FRANUS M, et al. Zeolite properties improvement by chitosan modification—Sorption studies[J]. Journal of Industrial and Engineering Chemistry, 2017, 52: 187-196. DOI:10.1016/j.jiec.2017.03.043 |
| [42] |
XIE J, LI C, CHI L, et al. Chitosan modified zeolite as a versatile adsorbent for the removal of different pollutants from water[J]. Fuel, 2013, 103: 480-485. DOI:10.1016/j.fuel.2012.05.036 |
| [43] |
TAUANOV Z, TSAKIRIDIS P E, MIKHALOVSKY S V, et al. Synthetic coal fly ash-derived zeolites doped with silver nanoparticles for mercury (Ⅱ) removal from water[J]. Journal of Environmental Management, 2018, 224: 164-171. |
| [44] |
BONETTI B, WALDOW E C, TRAPP G, et al. Production of zeolitic materials in pilot scale based on coal ash for phosphate and potassium adsorption in order to obtain fertilizer[J]. Environmental Science and Pollution Research, 2021, 28(3): 2638-2654. DOI:10.1007/s11356-020-11447-y |
| [45] |
ABDELLAOUI Y, ABOU OUALID H, HSINI A, et al. Synthesis of zirconium-modified Merlinoite from fly ash for enhanced removal of phosphate in aqueous medium: Experimental studies supported by Monte Carlo/SA simulations[J]. Chemical Engineering Journal, 2021, 404: 126600. DOI:10.1016/j.cej.2020.126600 |
| [46] |
李思洋. 粉煤灰基菱沸石的合成、改性及其气体吸附性能研究[D]. 沈阳: 东北大学, 2016 LI Siyang. Study on synthesis, modification and gas adsorption properties of fly ash-based chabazite[D]. Shenyang: Northeastern University, 2016 (in Chinese) |
| [47] |
DÍAZ E, ORDÓÑEZ S, VEGA A, et al. Catalytic combustion of hexane over transition metal modified zeolites NaX and CaA[J]. Applied Catalysis B: Environmental, 2005, 56(4): 313-322. DOI:10.1016/j.apcatb.2004.09.016 |
| [48] |
王宇, 谌建宇, 李小明, 等. 镧改性粉煤灰合成沸石的同步脱氨除磷研究[J]. 中国环境科学, 2011, 31(7): 1152-1158. WANG Yu, CHEN Jianyu, LI Xiaoming, et al. Stimultaneous removal of ammonium and phosphate in waste water by La-modified synthetic zeolite from coal fly ash[J]. China Environmental Science, 2011, 31(7): 1152-1158. (in Chinese) |
| [49] |
WANG Z, LU S, WU D, et al. Control of internal phosphorus loading in eutrophic lakes using lanthanum-modified zeolite[J]. Chemical Engineering Journal, 2017, 327: 505-513. DOI:10.1016/j.cej.2017.06.111 |
| [50] |
HOR K Y, CHEE J M C, CHONG M, et al. Evaluation of physicochemical methods in enhancing the adsorption performance of natural zeolite as low-cost adsorbent of methylene blue dye from wastewater[J]. Journal of Cleaner Production, 2016, 118: 197-209. DOI:10.1016/j.jclepro.2016.01.056 |
| [51] |
PAUL B, DYNES J J, CHANG W. Modified zeolite adsorbents for the remediation of potash brine-impacted groundwater: Built-in dual functions for desalination and pH neutralization[J]. Desalination, 2017, 419: 141-151. DOI:10.1016/j.desal.2017.06.009 |
| [52] |
SOH J C, CHONG S L, HOSSAIN S S, et al. Catalytic ethylene production from ethanol dehydration over non-modified and phosphoric acid modified zeolite H-Y (80) catalysts[J]. Fuel Processing Technology, 2017, 158: 85-95. DOI:10.1016/j.fuproc.2016.12.012 |
| [53] |
WANG S, PENG Y. Natural zeolites as effective adsorbents in water and wastewater treatment[J]. Chemical Engineering Journal, 2010, 156(1): 11-24. DOI:10.1016/j.cej.2009.10.029 |
| [54] |
ZHANG B, WU D, WANG C, et al. Simultaneous removal of ammonium and phosphate by zeolite synthesized from coal fly ash as influenced by acid treatment[J]. Journal of Environmental Sciences, 2007, 19(5): 540-545. DOI:10.1016/S1001-0742(07)60090-4 |
| [55] |
吕海亮, 王本红, 马毅. 粉煤灰合成Na-P1沸石去除饮用水中氟的研究[J]. 燃料化学学报, 2008, 36(6): 743-747. LV Hailiang, WANG Benhong, MA Yi. Removal of fluorine from drinking water with Na-P1 zeolite synthesized from coal fly ash[J]. Journal of Fuel Chemistry and Technology, 2008, 36(6): 743-747. (in Chinese) |
| [56] |
许莹, 陈韬宇, 蒋金龙. 磁性粉煤灰沸石的制备及其对Cu2+的吸附研究[J]. 非金属矿, 2014, 37(6): 62-65. XU Ying, CHEN Taoyu, JIANG Jinlong. Preparation of magnetic fly ash zeolite and its adsorption to Cu2+[J]. Non-Metallic Mines, 2014, 37(6): 62-65. (in Chinese) |
| [57] |
BELVISO C, AGOSTINELLI E, BELVISO S, et al. Synthesis of magnetic zeolite at low temperature using a waste material mixture: Fly ash and red mud[J]. Microporous and Mesoporous Materials, 2015, 202: 208-216. DOI:10.1016/j.micromeso.2014.09.059 |
| [58] |
YAMAURA M, FUNGARO D A. Synthesis and characterization of magnetic adsorbent prepared by magnetite nanoparticles and zeolite from coal fly ash[J]. Journal of Materials Science, 2013, 48(14): 5093-5101. DOI:10.1007/s10853-013-7297-6 |
| [59] |
YANG Y, ZHANG P, JIANG J, et al. Synthesis and properties of magnetic zeolite with good magnetic stability from fly ash[J]. Journal of Sol-Gel Science and Technology, 2018, 87(2): 408-418. DOI:10.1007/s10971-018-4733-8 |
| [60] |
SUGAWARA T, MATSUURA Y, ANZAI T, et al. Removal of ammonia nitrogen from water by magnetic zeolite and high-gradient magnetic separation[J]. IEEE Transactions on Applied Superconductivity, 2016, 26(4): 1-4. |
| [61] |
WU D, SUI Y, HE S, et al. Removal of trivalent chromium from aqueous solution by zeolite synthesized from coal fly ash[J]. Journal of Hazardous Materials, 2008, 155(3): 415-423. DOI:10.1016/j.jhazmat.2007.11.082 |
| [62] |
BELVISO C, CAVALCANTE F, DI GENNARO S, et al. Removal of Mn from aqueous solution using fly ash and its hydrothermal synthetic zeolite[J]. Journal of Environmental Management, 2014, 137: 16-22. |
| [63] |
李喜林, 张颖, 赵雪, 等. 粉煤灰合成沸石吸附含铬废水中3价铬的研究[J]. 非金属矿, 2017, 40(5): 93-95. LI Xilin, ZHANG Ying, ZHAO Xue, et al. Study on adsorption of trivalent chromium-containing wastewater by zeolite synthesized from coal fly ash[J]. Non-Metallic Mines, 2017, 40(5): 93-95. (in Chinese) |
| [64] |
王光辉, 李敏, 郭峰, 等. 粉煤灰合成沸石的吸附性能及其机理研究[J]. 中国资源综合利用, 2017, 35(9): 35-39. WANG Guanghui, LI Min, GUO Feng, et al. Study on adsorption performance and mechanism of synthetic zeolite from fly ash[J]. China Resources Comprehensive Utilization, 2017, 35(9): 35-39. (in Chinese) |
| [65] |
PRABHAKAR R, SAMADDER S R. Effective immobilization and reduction in bioavailability of Cd in a L. succinea growing in contaminated sediment by the application of alkali synthesized fly ash based zeolite (FABZ)[J]. Microporous and Mesoporous Materials, 2020, 306: 110416. DOI:10.1016/j.micromeso.2020.110416 |
| [66] |
ZHANG Y, CHEN Y, KANG W, et al. Excellent adsorption of Zn(Ⅱ) using NaP zeolite adsorbent synthesized from coal fly ash via stage treatment[J]. Journal of Cleaner Production, 2020, 258: 120736. DOI:10.1016/j.jclepro.2020.120736 |
| [67] |
王曦, 张雪峰, 阙耀华, 等. 粉煤灰水热法合成沸石及其对氨氮吸附性能的研究[J]. 环境工程, 2012, 30(5): 13-16. WANG Xi, ZHANG Xuefeng, QUE Yaohua, et al. Study on synthesis of zeolites from fly ash by hydrothermal method and its adsorption performance of ammonia nitrogen[J]. Environmental Engineering, 2012, 30(5): 13-16. (in Chinese) |
| [68] |
LIU M, XI B, HOU L, et al. Magnetic multi-functional nano-fly ash-derived zeolite composites for environmental applications[J]. Journal of Materials Chemistry A, 2013, 1(40): 12617-12626. DOI:10.1039/c3ta13180g |
| [69] |
GOLLAKOTA A R K, VOLLI V, MUNAGAPATI V S, et al. Synthesis of novel ZSM-22 zeolite from Taiwanese coal fly ash for the selective separation of Rhodamine 6G[J]. Journal of Materials Research and Technology, 2020, 9(6): 15381-15393. DOI:10.1016/j.jmrt.2020.10.070 |
| [70] |
HOSSINI ASL S M, MASOMI M, TAJBAKHSH M. Hybrid adaptive neuro-fuzzy inference systems for forecasting benzene, toluene & m-xylene removal from aqueous solutions by HZSM-5 nano-zeolite synthesized from coal fly ash[J]. Journal of Cleaner Production, 2020, 258: 120688. DOI:10.1016/j.jclepro.2020.120688 |
| [71] |
DONG Y, WU D, CHEN X, et al. Adsorption of bisphenol A from water by surfactant-modified zeolite[J]. Journal of Colloid and Interface Science, 2010, 348(2): 585-590. DOI:10.1016/j.jcis.2010.04.074 |
| [72] |
HEDAYATI M S, LI L. Removal of polycyclic aromatic hydrocarbons from aqueous media using modified clinoptilolite[J]. Journal of Environmental Management, 2020, 273: 111113. DOI:10.1016/j.jenvman.2020.111113 |
| [73] |
Li X, Kuang Y, Chen J, et al. Competitive adsorption of phosphate and dissolved organic carbon on lanthanum modified zeolite[J]. J Colloid Interface Sci, 2020, 574: 197-206. DOI:10.1016/j.jcis.2020.04.050 |
| [74] |
CHEN J, KONG H, WU D, et al. Removal of phosphate from aqueous solution by zeolite synthesized from fly ash[J]. Journal of Colloid and Interface Science, 2006, 300(2): 491-497. DOI:10.1016/j.jcis.2006.04.010 |
| [75] |
梁凤焦, 李多松, 邓霞. HDTMA改性粉煤灰沸石对水中铬酸盐的吸附[J]. 环境科学与管理, 2008, 33(6): 50-52. LIANG Fengjiao, LI Duosong, DENG Xia. Adsorption of chromate onto HDTMA-zeolitized fly ash in water[J]. Environmental Science and Management, 2008, 33(6): 50-52. (in Chinese) |
| [76] |
MOKGEHLE T M, RICHARDS H, CHIMUKA L, et al. Sulphates removal from AMD using CFA hydrothermally treated zeolites in column studies[J]. Minerals Engineering, 2019, 141: 105851. |
| [77] |
REDDY A G K, YULIM C, PRASANNA L L, et al. Facile synthesis of economical feasible fly ash-based zeolite-supported nano zerovalent iron and nickel bimetallic composite for the potential removal of heavy metals from industrial effluents[J]. Chemosphere, 2020. DOI:10.1016/j.chemosphere.2020.128889 |
| [78] |
GOSCIANSKA J, PTASZKOWSKA-KONIARZ M, FRANKOWSKI M, et al. Removal of phosphate from water by lanthanum-modified zeolites obtained from fly ash[J]. Journal of Colloid and Interface Science, 2018, 513: 72-81. |
| [79] |
VOLLI V, PURKAIT M K. Selective preparation of zeolite X and A from flyash and its use as catalyst for biodiesel production[J]. Journal of Hazardous Materials, 2015, 297: 101-111. |
| [80] |
PAVLOVIĆ S, MARINKOVIĆ D, KOSTIC M, et al. A CaO/zeolite-based catalyst obtained from waste chicken eggshell and coal fly ash for biodiesel production[J]. Fuel, 2020, 267: 117171. |
| [81] |
BOYCHEVA S, ZGUREVA D, VÁCLAVÍKOVÁ M, et al. Studies on non-modified and copper-modified coal ash zeolites as heterogeneous catalysts for VOCs oxidation[J]. Journal of Hazardous Materials, 2019, 361: 374-382. |
| [82] |
POPOVA M, BOYCHEVA S, LAZAROVA H, et al. VOC oxidation and CO2 adsorption on dual adsorption/catalytic system based on fly ash zeolites[J]. Catalysis Today, 2020, 357: 518-525. |
| [83] |
黄宇玫, 欧阳小平, 蒋柏泉. CuO/沸石催化剂制备的工艺优化及其应用[J]. 应用化工, 2019, 48(12): 2894-2899. HUANG Yumei, OUYANG Xiaoping, JIANG Boquan. Process optimization and application of CuO/zeolite catalyst preparation[J]. Applied Chemical Industry, 2019, 48(12): 2894-2899. (in Chinese) |
| [84] |
PARK J, CHOE J K, LEE W, et al. Highly fast and selective removal of nitrate in groundwater by bimetallic catalysts supported by fly ash-derived zeolite Na-X[J]. Environmental Science: Nano, 2020, 7(11): 3360-3371. |
| [85] |
Subbulekshmi N L, Subramanian E. Nano CuO immobilized fly ash zeolite Fenton-like catalyst for oxidative degradation of p-nitrophenol and p-nitroaniline[J]. Journal of Environmental Chemical Engineering, 2017, 5(2): 1360-1371. |
| [86] |
HLEKELELE L, FRANKLYN P J, DZIIKE F, et al. Novel synthesis of Ag decorated TiO2 anchored on zeolites derived from coal fly ash for the photodegradation of bisphenol-A[J]. New Journal of Chemistry, 2018, 42(3): 1902-1912. |
 2023, Vol. 40
2023, Vol. 40