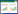农用化学品是指化肥、化学农药以及用于农业的植物生长激素组成的化学产品[1]。其中,化肥可以通过提供作物所需的养分来提高作物的质量和产量[2];农药通过最大限度地减少或消除害虫、真菌以及杂草等负面生物造成的威胁来提高单位面积的粮食生产力[3]。此外,一些公共卫生问题也需要使用强效杀虫剂来解决[4]。根据联合国粮农组织(FAO)的预测[5],到2050年,全球农业生产必须增加70%,才能满足不断增长人口的需求,而这需要通过提高可耕作农田的产量和作物产量水平来实现。因而,农用化学品的广泛应用对于人类社会的进步与发展具有重要意义。
然而,农药和化肥会造成一系列不可忽视的环境和人类健康危害。例如,双对氯苯基三氯乙烷(DDT)的广泛使用导致美国国鸟白头海雕几乎灭绝;1986年Sandoz化学品泄漏造成莱茵河污染灾难,造成约60万条鱼死亡,并迫使沿海供水设施和啤酒厂关闭;以及2017年氟虫腈引发的“毒鸡蛋”事件对人类健康构成了极大威胁。化肥的大量使用对地下水也有不利影响,如严重损害了海洋生态环境,造成水生生物的大量死亡。此外,某些形式的氮肥以气体形式排放,导致严重的酸雨和气候变化。这些严重的环境污染促使我们考虑一个基本问题:如何发展有效且环境可接受的绿色农业?
从先导化合物出发,开发优质的新型农药可能是一个有效的策略。2010—2014年间,向市场推出新农药活性组分的平均成本为2.86亿美元,比1995年增加了约1.34亿美元。此外,从产品发现到上市的平均时间约为11.3年,比1995年增加了4年。因此,从先导化合物的筛选到最终产品功效和毒性的评估通常需要耗费大量的时间和精力,如何对目前正在使用的存在缺陷的农药活性组分进行性质改善以使其更好地发挥作用可能是一个重点值得关注的问题。
化合物可以多种固态形式存在,由于分子间相互作用的差异,影响内能和焓,以及无序程度,从而影响熵[6, 7]。因此,它们的物理化学性质通常存在很大差异特性。有机共晶工程指的是通过对超分子化学原理的理解,并将其用于构建具有所需物理和化学特性的新固体的一种有效的固体化学策略[8]。其具体的定义为:“共晶体是固态的晶体单相材料,通常由2种或更多种不同的分子和/或离子化合物以化学计量比组成,既不是溶剂化物也不是简单盐。”
虽然关注度不及活性药物成分,但农用化学品的共晶形态仍得到充分利用,如经典的市售共晶型杀菌剂嘧霉胺和二氢蒽醌形成的共晶具有良好的协同和缓释作用[9]。最近,Olenik等[10]进一步强调“有机固态在现代农业化学中越来越受到科学的关注”,并倡导多晶型在现代作物保护中的重要性。因此,深入了解并进一步利用有机共晶工程策略,让当前存在缺陷的农用化学品焕发出新鲜的魅力,至关重要。
为突出有机共晶工程策略在绿色农业中的价值和发展趋势,本综述以农药、化肥以及精油等3类农用化学品为研究对象,通过具体的应用场景突出了农用化学品共晶缓释技术的发展历程,并在上述分析总结的基础上,提出了有机共晶工程策略在未来绿色农业中的发展前景和挑战。本综述旨在提高学者对固态化学在农业发展中应用的认识,旨在促进未来生产更高效、更生态友好的农用化学品。
1 农用化学品的共晶缓释技术 1.1 化学农药的共晶缓释技术90%的农药通过常规使用后无法到达其目标位点以发挥作用,并且通过蒸发、降解、浸出和径流进入环境,造成地表水和地下水污染等严重不利影响[11]。因此,控制化学农药的释放速率目前已被广泛重视,目前常用的手段包括通过各种载体,包括有机黏土[12]、藻酸盐[13]、刺激响应水凝胶[14]、多孔碳酸钙微球[15]、明胶微球[16]、工程纳米金属[17]和共价有机框架[18]等的负载策略,但是这些方法通常操作繁琐且难以放大,而通过简单的共晶制备以实现缓释可能是一个新的有效策略。
新烟碱类杀虫剂作为广泛应用的高效农药,已广泛应用于防治蚜虫、蓟马和白粉虱等多种害虫,然而其高水溶性使其利用效率降低,通常采用的高浓度施用的解决方案使得其潜在的农药毒性问题被放大。啶虫脒-氰虫酰胺共晶[19]通过在晶格中引入氯虫苯甲酰胺,不仅在杀虫活性上达到了协同增效的目的,更为重要的是,其对蜜蜂的毒性得以降低。
苯嗪草酮是一种甜菜田中常用的芽前和芽后选择性除草剂,因其水溶性高而在世界范围内的地下水中造成较大的污染。通过将其与2种黄酮类化合物通过超分子化学的方式组装成共晶[图 1(a)][20],其溶出速率[图 1(b)]实现了较大的降低。土柱淋溶实验[图 1(c)]证实了苯嗪草酮缓释共晶相较于纯苯嗪草酮原药在土壤中有更好的保留能力,并且在模拟降雨的条件下依旧能够维持很好的除草活性[图 1(d)~图 1(e)]。更进一步的,Xiao等[20]通过相关的分子动力学模拟对共晶实现缓释的机理进行了相应的分析。具体而言,共晶的释放涉及体系的吉布斯自由能变化,不仅与晶格破坏的能量变化相关,还涉及到与溶剂化有关的能量变化。相关的模拟计算表明,苯嗪草酮与2种黄酮类化合物形成的共晶具有增强的晶格能与降低的溶剂化自由能,使之延缓了除草剂原药的释放。

|
| 图 1 (a) 苯嗪草酮与肉桂酸、柚皮素和黄芩素形成共晶的晶体结构图;(b)苯嗪草酮及其3种共晶在25 ℃下在水中的溶出曲线;(c) 苯嗪草酮及其3种共晶在土柱中的穿透曲线;(d) MET和3种共晶体处理的肯塔基早熟禾的代表性形态图片和(e)计算的鲜质量损失数据[20] Fig.1 (a) Crystal structure of the cocrystals of metamitron with cinnamic acid, naringenin and baicalein; (b) Dissolution profiles of metamitron, and three cocrystals in water at 25 ℃; (c) Breakthrough curves of metamitron, and three cocrystals in soil columns. Representative morphological pictures (d) and calculated FWR data (e) of Kentucky bluegrass treated by MET and three cocrystals[20] |
| |
杀菌剂甲霜灵在水中具有高达8.4 g ·L-1(25 ℃)的溶解度,这使得其在用于治疗瓜果等作物的霜霉病菌、疫霉病菌和腐病菌时,需要多次重复施药,这不仅大大提高了防治成本而且加重了对地下水的污染。研究表明,将甲霜灵与丙硫菌唑制备成共晶[21],使得甲霜灵在植物中的保留时间更长,同时还减少了对地下水的长期渗透。此外,由甲基托布津和戊唑醇等三唑类杀菌剂组装的共晶[22]具有改变活性成分溶解特性的能力,从而可用作农业和园艺缓释制剂的原料。
1.2 化学肥料的共晶缓释技术化肥对全球农业和粮食生产至关重要[23-26],尿素[27, 28]作为含氮量最高(约46%)的固体氮肥,已被广泛使用。然而,研究表明[29],作物仅吸收了47% 的施肥氮,其余的因浸出、挥发和水解等各种因素而流失。这些行为不仅降低了氮肥使用的有效性,而且对水资源[30, 31]和大气[32, 33]产生了不可逆转的负面影响。目前较为成熟的解决方案[34-36]是使用纳米结构材料作为肥料控释载体构建“智能肥料”,以最佳时间和剂量为作物提供养分。尽管如此,有机晶体工程在这一研究领域仍保持着它的特殊魅力,尿素是其中的“故事主角”。
尿素共晶的制备大多是基于机械化学的,主要包括干磨[37]、液体辅助研磨[38]、离子液体辅助研磨[39]和聚合物辅助研磨[40]。根据配体的类型,其可以分为离子共晶或中性共晶。我们在表 1中总结了迄今为止主要关于尿素缓释共晶体的研究,并对代表性案例进行了详细回顾。
| 案例 | 类型 | 制备方法 | 结论 | 参考文献 |
| CaSO4·4CO(NH2)2 | 离子共晶 | 在水存在下研磨、压实和混合 | 硬度好,吸湿性低 | [47] |
| CaSO4·4CO(NH2)2, Ca(H2PO4)2·4CO(NH2)2, Ca(NO3)2·4CO(NH2)2, MgSO4·6CO(NH2)2·0.5H2O, Mg(H2PO4)2·4CO(NH2)2, and Mg(NO3)2·4CO(NH2)2·xH2O | 离子共晶 | 干磨法 | 与纯尿素相比,能够减少NH3排放到环境中 | [48] |
| (urea·tetra-methyl(N-oxide)pyrazine, urea·tetra-methyl bis(N, N-oxide)pyrazine, urea·pimelic acid, and urea·4-nitrophenod) | 中性共晶 | 溶剂辅助研磨与水热法 | 溶解度降低(尿素·庚二酸为40倍,尿素·4-硝基苯酚为60倍),可保持1个月良好的湿度稳定性 | [41] |
| urea·catechol (1∶1) | 中性共晶 | 溶剂辅助研磨与缓慢挥发溶剂 | 增加对脲酶的抑制活性,减少水蒸气吸附3.5倍,降低溶解度15% | [49] |
| CaSO4·4CO(NH2)2, Ca(H2PO4)2·4CO(NH2)2, Ca(NO3)2·4CO(NH2)2, MgSO4·6CO(NH2)2·0.5H2O, Mg(H2PO4)2·4CO(NH2)2, and Mg(NO3)2·4CO(NH2)2·2H2O | 离子共晶 | 干磨法 | 降低尿素的水解,从而潜在地减少NH3的损失 | [50] |
| urea·ZnCl2·KCl (form Ⅰ & Ⅱ) | 离子共晶 | 溶剂辅助研磨 | 溶解度降低20%,大大抑制脲酶活性 | [51] |
| Zn(thiourea)(urea)Cl2 | 离子共晶 | 溶剂辅助研磨和浆液法 | 溶解度降低1/3,吸湿性和加速稳定性增加,对脲酶和氨单加氧酶的抑制活性增强 | [42] |
| Ca[CO(NH2)2]4(H2PO4)2 | 离子共晶 | 溶剂辅助研磨 | 不仅加深了我们对机械化学基础和机制的理解,而且还展示了如何利用水基自催化来显著简化离子共晶的产生 | [52] |
| CaSO4·4urea | 离子共晶 | 干磨法 | 从土壤中淋溶出的尿素量显着减少,可提高玉米产量 | [53] |
| urea·salicylic acid (1∶1) | 中性共晶 | 干磨法 | 提高水解稳定性并有助于减少NH3排放 | [54] |
| urea·adipic acid form Ⅱ (2∶1) | 中性共晶 | 溶剂辅助研磨 | 溶解度降低了96.8%,对相对湿度的稳定性增强,尿素释放行为减慢,浸出量也减少 | [43] |
| 2ZnO·3B2O3·7H2O | 离子共晶 | 溶剂辅助研磨 | 合成含锌(Zn) 和硼(B) 的缓释微量营养素肥料 | [44] |
| CaNH4PO4·H2O, MgNH4PO4·6H2O, Ca(NH4)2(HPO4)2·H2O dimorph B, and Mg(NH4)2(HPO4)2·4H2O dimorph A | 离子共晶 | 溶剂辅助研磨 | 获得吸湿性极低的结晶复合肥 | [45] |
Sandhu等[41]使用来自剑桥结构数据库的完整相互作用图和相关数据将尿素中的相互作用类型分为五个不同的合成子[图 2(a)],并有效地确定了60个共形成体中的49个新相。其中,尿素∶庚二酸和尿素∶4-硝基苯酚共晶的溶解度分别比纯尿素低23和66倍。此外,在85%的湿度下,尿素在不到24 h转变为透明液体,而2种共晶体在1个多月内仍保持结晶固体形态[图 3(b)]。新型三元Zn(Ⅱ)-硫脲-脲离子共晶体[42]的设计是提高尿素利用效率的又一例证。它不仅使得尿素的溶解度降低1/3[图 2(e)],还使得其加速和吸湿稳定性[图 2(f)]有所提高。更为关键的是,它还能很好地抑制氮循环中的2种关键酶:脲酶和巴氏芽孢杆菌[图 2(g), 图 2(f)], 从而提高土壤氮肥的利用效率。

|
| 图 2 (a) 尿素共晶的五种超分子合成子; (b) 尿素-庚二酸和尿素-4-硝基苯酚对85%湿度的响应[55];(c)尿素-己二酸多晶型Ⅰ和Ⅱ的晶体结构; (d)尿素·己二酸Ⅱ型(红色)、Ⅰ型和Ⅱ型的混合物(黑色)和商品尿素(蓝色)的释放曲线[43];(e) ZnU, ZnT以及ZnTU在水中的溶解度对比; (f) 尿素, ZnTU以及硫脲的吸附-解析行为曲线; (g) 脲酶(JBU) 在pH 7.5时随ZnTU浓度的增加的活性变化; (h) 巴氏芽孢杆菌在8 μmol ·L-1 ZnTU、ZnCl2或硫脲处理下的氨化百分比和氧气消耗百分比[42] Fig.2 (a) Five synthons in cocrystals of urea; (b) Response to 85% humidity of urea: pimelic acid and urea: 4-nitrophenol[43]; (c) Crystal structures of polymorphs Ⅰ and Ⅱ of urea-adipic acid; (d) Urea release profile of urea ·adipic acid Form Ⅱ (red), a mixture of Form Ⅰ & Ⅱ (black), and commercial urea (blue)[43]; (e) Comparison of ZnU, ZnT, and ZnTU solubility in water; (f) Adsorption/desorption branches of RH on urea, ZnTU, and thiourea; (g) Residual percentage activity of jack bean urease (JBU) at pH 7.5, as a function of increasing concentrations of ZnTU; (h) Percentage ammonification and percentage O2 consumption by S pasteurii, at pH 7.5, as a function of 8 μmol ·L-1 ZnTU, ZnCl2 or thiourea[42] |
| |

|
| 图 3 由精油的3种成分(百里酚、香芹酚和丁香酚)和2种配体(吩嗪和六亚甲基四胺)组装的共晶1~6的示意图; (b)在30 ℃(左)和4 ℃(右)下,纯精油组分(实线)和共晶(虚线)的精油释放曲线[63]; (c) 纯EOs活性成分的释放曲线(实线)与包装原型中活性成分的释放曲线(虚线); (d) 对包装在低密度聚乙烯中的葡萄进行感官评价,对照(蓝色),共晶活性组分(红色)[65] Fig.3 Schematic representation of the cocrystals 1—6 synthesized from the combination of three constituents of essential oil (thymol, carvacrol and eugenol) and two coformers (phenazine and hexamethylenetetramine); (b) Essential oil release profile from the pure substance (plain line) and from the cocrystals (dashed and dotted lines) at 30 ℃ (left) and 4 ℃ (right)[63]; (c) Release profile of the active components from pure EOs (solid line) vs. release profile of the active components from the packaging prototypes (dashed line); (d) Sensory evaluation of grapes packed in non-functionalized LDPE used as control (blue) and in the developed active prototypes (red)[65] |
| |
此外,Parakatawella等[43]发现了尿素-己二酸(2∶1)共晶的晶型Ⅱ[图 2(c)],溶解度比纯尿素低96.8%,浸出率也降低了约1.8倍[图 2(d)],这一研究结果使得通过有机共晶工程的策略可以很好的提高尿素的利用效率并降低浸出所带来的环境危害。
除了改善氮肥的功效,有机共晶工程还可用于提高其他类型肥料的功效。如表 1所述,Zheng等[44]使用机械化学开发了一种含有锌和硼的缓释微量营养素肥料(离子共晶体),而Sharma等[45]将初级营养元素(N和P)和次级营养元素(Mg、Ca)进行组装,研究表明复盐的形成可以很好的提高吸湿稳定性。此外,合成氨(NH3)是一种用于制造主要氮肥尿素的反应物,消耗了全球高达1%的能源和约4%的天然气[46]。通过有机共晶工程制备的复盐可以使得养分以较慢的速率释放到环境中,从而减少矿物肥料流入的需求以降低其生产过程中消耗的能量。
1.3 精油(EOs)的共晶缓释技术化学农药的使用量势必会对生态系统产生不利影响,因此,开发环保及可持续的绿色农药已成为未来发展的必然趋势。精油(EOs) 是在植物中发现的次生代谢物,由于它们在植物防御系统中对微生物、昆虫、食草动物和化感作用相互作用的关键作用,已成为开发绿色农药的一种新策略[56, 57]。
虽然EOs具有许多优点,如高活性和安全性,但它也有一些缺点,包括低水溶性[58, 59]和高挥发性[60-62],这极大限制了它们的应用。因此,确定如何控制EO的释放以最大限度地提高其有效性是一项重要的挑战。
在2019年,Mazzeo等[63]设计了一个基于图 3(a)中的EOs成分的共晶体调色板,并使用丁香酚、香芹酚和百里香酚等3种低熔点植物精油作为模型物质。以吩嗪和六亚甲基四胺作为配体,通过绿色的机械研磨成功地制备了EOs的7种共晶。环境释放实验[图 3(b)]表明,共晶体比纯EOs具有更大的长期释放能力,并且生物测定显示所制备的精油共晶对6种细菌和3种真菌植物病原菌的活性并无损失。此外,作者从分子模拟的角度进行了释放速率的理论预测,证实了分子间相互作用能量对是共晶的最佳关联因素,这比实验熔点具有更强的关联性。进一步,又针对于香芹酚和百里酚开发出了其与异烟酰胺、吡嗪、2, 3, 5, 6-四甲基吡嗪和2, 3-二甲基喹喔啉等4种配体的8种共晶[64],通过顶空气相色谱-质谱(GC-MS) 分析了所有共晶在14 d内的释放行为并通过PXRD进行了稳定性测试,证实了共晶相较于精油自身存在着延缓释放的能力[图 3(c)]。更为有趣的是,百里酚-吡嗪共晶表现出了共晶多晶型的行为,其在释放过程中向更稳定的晶型转变,这对于延迟EOs释放从而提高食品保存效率可能非常有用。
基于这一研究,Bianchi等[65]考虑了另一个有趣且有价值的问题:可以生产哪些新的活性食品包装材料来延长食品的保质期以防止浪费?其解决方案是:3种EOs[66, 67](香芹酚、百里酚和肉桂醛)的共结晶。生测实验表明通过精油与GRAS中的多种可药用配体构建共晶可以提高抑菌活性[69%(±15%)],从而可以很好抑制水果和蔬菜变质。进一步的感官评估[图 3(d)]显示,在室温下储存的葡萄样品的保质期长达7 d。本研究成果基于“质量源于设计”的原则,为有机共晶工程在食品保鲜中的应用提供了极大的便利。
2 结论与展望根据联合国环境署的绿色经济报告[68],绿色农业是指越来越多地使用农业实践和技术,同时保持和提高生产力和盈利能力,确保粮食和生态系统的可持续性;减少负外部性(例如排放)并逐渐增加正外部性(例如碳汇或生物多样性)。
如上所述,有机共晶工程策略可以通过超分子化学的原理开发出新的固体形式应用于未来的生态友好型农业,随着研究的不断深入,其应用范围也在不断扩大。更进一步,以下前景和挑战值得我们关注和重视。
2.1 配体的安全性农药共晶的缓释效果的实现很大程度上取决于配体的选择,因而配体的环境安全性是值得重点关注的。尤其当作物经历降雨或者灌溉时,配体分子可能发生解离并进入地下水中,因而选择对靶标作物及其他环境生物安全的配体是首先需要考虑的问题。
2.2 共晶实现缓释的机理目前尽管有较多的案例已经成功的证实了共晶的形成可以实现农药的缓释,但是具体的机理并不明确。可能是由于形成的共晶暴露了更少的氢键位点以降低释放速率?或者配体分子与农药分子在晶格中以更强大的氢键结合,从而具有更强的晶格能?又或者配体分子在溶液中与农药分子依旧能够保持很好的结合能力,从而降低了农药分子与水分子的溶剂化位点,而抑制其释放?这值得进一步从实验与模拟的角度进行进一步的探究。
2.3 农药共晶的剂型众所周知,剂型是农药使用的载体,因此为缓释的农药共晶选择合适的载体至关重要。当制备形成共晶后,通常农药活性组分的溶解度会发生较大的变化,因而可能适用于原药的真溶液剂型需要替换为水悬浮剂或者水分散粒剂。因而,进一步开发缓释的农药共晶的剂型是使其走向市场并提高原有农药生命周期的有效手段。
作为一种能有效实现缓释的手段,有机共晶工程在化学农药、化肥以及精油的缓释方面发挥着越来越重要的作用,一方面可以极大地提高利用效率,另一方面也可以减少农用化学品的施用所带来的环境污染问题。在未来,理论预测与实验相结合的方式将会使更多的具有优异性质的农用化学品共晶被开发出来,并且作为一种绿色的“活性组分”使得绿色农业得到更快更好地发展。
| [1] |
CLARKE E D, DELANEY J S. Physical and molecular properties of agrochemicals: An analysis of screen inputs, hits, leads, and products[J]. CHIMIA, 2003. DOI:10.2533/000942903777678641 |
| [2] |
TISDALE S L, NELSON W L. Soil fertility and fertilizers[J]. Soil Science, 1966. DOI:10.1007/s12155-019-10021-w |
| [3] |
COOPER J. The benefits of pesticides to mankind and the environment[J]. Crop Protection, 2007, 26(9): 1337-1348. DOI:10.1016/j.cropro.2007.03.022 |
| [4] |
ROGAN W J, CHEN A. Health risks and benefits of bis(4-chlorophenyl)-1, 1, 1-trichloroethane (DDT)[J]. The Lancet, 2005, 366(9487): 763-773. DOI:10.1016/S0140-6736(05)67182-6 |
| [5] |
OTEKUNRIN O A, SAWICKA B, ADEYONU A G, et al. Cocoyam[colocasia esculenta (L.) schott]: Exploring the production, health and trade potentials in sub-Saharan Africa[J]. Sustainability, 2021. DOI:10.3390/su13084483 |
| [6] |
KHANKARI R K, GRANT D. Pharmaceutical hydrates[J]. Thermochimica Acta, 1995, 248: 61-79. DOI:10.1016/0040-6031(94)01952-D |
| [7] |
GRANT D J. Theory and origin of polymorphism[J]. Polymorphism in Pharmaceutical Solids, 1999, 1-34. |
| [8] |
SUN L, ZHU W, ZHANG X, et al. Creating organic functional materials beyond chemical bond synthesis by organic cocrystal engineering[J]. Journal of the American Chemical Society, 2021, 143(46): 19243-19256. DOI:10.1021/jacs.1c07678 |
| [9] |
SEKHON B S. Co-crystals of agrochemical actives[J]. International Journal of Agricultural Sciences, 2014, 5(3): 472-475. |
| [10] |
OLENIK B, KEIL B, JESCHKE P. Importance of chemical polymorphism in modern crop protection[J]. Pest Management Science, 2022, 78(7): 2746-2758. DOI:10.1002/ps.6919 |
| [11] |
SMITH A M, GILBERTSON L M. Rational ligand design to improve agrochemical delivery efficiency and advance agriculture sustainability[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(11): 13599-13610. |
| [12] |
HERMOSIN M, CELIS R, FACENDA G. Bioavailability of the herbicide 2, 4-D formulated with organoclays[J]. Soil Biology and Biochemistry, 2006, 38(8): 2117-2124. DOI:10.1016/j.soilbio.2006.01.032 |
| [13] |
SUNITA P, JAYA B, RAJESH S, et al. Sustained release of pesticide (Cypermethrin) from nanocarriers: An effective technique for environmental and crop protection[J]. Process Safety and Environmental Protection, 2018, 117: 315-325. DOI:10.1016/j.psep.2018.05.012 |
| [14] |
XIANG Y, LU X, YUE J, et al. Stimuli-responsive hydrogel as carrier for controlling the release and leaching behavior of hydrophilic pesticide[J]. Science of the Total Environment, 2020. DOI:10.1016/j.scitotenv.2020.137811 |
| [15] |
XIANG Y, HAN J, ZHANG G, et al. Efficient synthesis of starch-regulated porous calcium carbonate microspheres as a carrier for slow-release herbicide[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(3): 3649-3658. |
| [16] |
ZHANG Q, DU Y, YU M, et al. Controlled release of dinotefuran with temperature/pH-responsive chitosan-gelatin microspheres to reduce leaching risk during application[J]. Carbohydrate Polymers, 2022. DOI:10.1016/j.carbpol.2021.118880 |
| [17] |
SALAM S T, PIRZADAH T B, DAR P A. Nanotechnology: An overview[M]//Nanotechnology in the Life Sciences. Cham: Springer International Publishing, 2020: 1-14
|
| [18] |
DENG X, ZHAO P, ZHOU X, et al. Excellent sustained-release efficacy of herbicide quinclorac with cationic covalent organic frameworks[J]. Chemical Engi neering Journal, 2021. DOI:10.1016/j.cej.2020.126979 |
| [19] |
ITO A, AMANO T, MASAKI R. Cocrystal of diamide insecticide and neonicotinoid insecticide, manufacture thereof, agrochemical formulation and seed treatment agent containing the cocrystal, and method for coating seed, WO2015072355[P/OL]. 2015
|
| [20] |
XIAO Y, WU C, ZHOU L, et al. Cocrystal engineering strategy for sustained release and leaching reduction of herbicides: A case study of metamitron[J]. Green Chemistry, 2022, 24(20): 8088-8099. DOI:10.1039/D2GC02949A |
| [21] |
FRIZZELL D. Metalaxyl and prothioconazole cocrystals and methods of making and using: US9795137[P]. 2017-10-24
|
| [22] |
YAMAMURA S. Manufacture of cocrystal of thiophanate methyl and triazole compound and agrochemical composition containing the cocrystal, WO2015093367[P/OL]. 2015
|
| [23] |
CHRISTIANSON C B, VLEK P L G. Alleviating soil fertility constraints to food production in West Africa: Efficiency of nitrogen fertilizers applied to food crops[J]. Fertilizer Research, 1991, 29(1): 21-33. DOI:10.1007/BF01048986 |
| [24] |
INGESTAD T. Nitrogen and plant growth; maximum efficiency of nitrogen fertilizers[J]. Ambio, 1977, 6(2/3): 146-151. |
| [25] |
FIXEN P E, WEST F B. Nitrogen fertilizers: Meeting contemporary challenges[J]. Ambio, 2002, 31(2): 169-176. DOI:10.1579/0044-7447-31.2.169 |
| [26] |
INDIRA C. Effect of nitrogen fertilizers on growth, yield and quality of hybrid rice (oryza sativa)[J]. Journal of Central European Agriculture, 2006, 6(4): 611-618. |
| [27] |
TEMPLEMAN W. Urea as a fertilizer[J]. The Journal of Agricultural Science, 1961, 57(2): 237-239. DOI:10.1017/S0021859600047717 |
| [28] |
ANKUMAH R O. The influence of source and timing of nitrogen fertilizers on yield and nitrogen use efficiency of four sweet potato cultivars[J]. Agriculture, Ecosystems & Environment, 2003, 100(2/3): 201-207. |
| [29] |
GALLOWAY J N, COWLING E B. Reactive nitrogen and the world: 200 years of change[J]. Ambio, 2002, 31(2): 64-71. DOI:10.1579/0044-7447-31.2.64 |
| [30] |
WANG F, ALVA A K. Leaching of nitrogen from slow-release urea sources in sandy soils[J]. Soil Science Society of America Journal, 1996, 60(5): 1454-1458. DOI:10.2136/sssaj1996.03615995006000050024x |
| [31] |
SCHERGER L E, ZANELLO V, LEXOW C. Impact of urea and ammoniacal nitrogen wastewaters on soil: Field study in a fertilizer industry (bahía Blanca, Argentina)[J]. Bulletin of Environmental Contamination and Toxicology, 2021, 107(3): 565-573. DOI:10.1007/s00128-021-03280-x |
| [32] |
RAWLUK C D L, GRANT C A, RACZ G J. Ammonia volatilization from soils fertilized with urea and varying rates of urease inhibitor NBPT[J]. Canadian Journal of Soil Science, 2001, 81(2): 239-246. DOI:10.4141/S00-052 |
| [33] |
COSKUN D, BRITTO D T, SHI W, et al. Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition[J]. Nature Plants, 2017. DOI:10.1038/nplants.2017.74 |
| [34] |
SUBRAMANIAN K S, MANIKANDAN A, THIRUNAVUKKARASU M, et al. Nano-fertilizers for balanced crop nutrition[M]//Nanotechnologies in Food and Agriculture. Cham: Springer International Publishing, 2015: 69-80
|
| [35] |
QURESHI A, SINGH D K, DWIVEDI S. Nano-fertilizers: A novel way for enhancing nutrient use efficiency and crop productivity[J]. International Journal of Current Microbiology and Applied Sciences, 2018, 7(2): 3325-3335. DOI:10.20546/ijcmas.2018.702.398 |
| [36] |
MADZOKERE T, MUROMBO L, CHIRIRIWA H. Nano-based slow releasing fertilizers for enhanced agricultural productivity[J]. Materials Today: Proceedings, 2021, 45: 3709-3715. DOI:10.1016/j.matpr.2020.12.674 |
| [37] |
TUMANOV I A, MICHALCHUK A A L, POLITOV A A, et al. Inadvertent liquid assisted grinding: A key to "dry" organic mechano-co-crystallisation?[J]. CrystEngComm, 2017, 19(21): 2830-2835. DOI:10.1039/C7CE00517B |
| [38] |
YING P, YU J, SU W. Liquid-assisted grinding mechanochemistry in the synthesis of pharmaceuticals[J]. Advanced Synthesis and Catalysis, 2021, 363(5): 1246-1271. DOI:10.1002/adsc.202001245 |
| [39] |
EKSILER K, ANDOU Y, YILMAZ F, et al. Dynamically controlled fibrillation under combination of ionic liquid with mechanical grinding[J]. Journal of Applied Polymer Science, 2017. DOI:10.1002/app.44469 |
| [40] |
FRIŠČIĆ T, MOTTILLO C, TITI H M. Mechanochemistry for synthesis[J]. Angewandte Chemie (International Ed in English), 2020, 59(3): 1018-1029. DOI:10.1002/anie.201906755 |
| [41] |
SANDHU B, SINHA A S, DESPER J, et al. Modulating the physical properties of solid forms of urea using co-crystallization technology[J]. Chemical Communications, 2018, 54(37): 4657-4660. DOI:10.1039/C8CC01144C |
| [42] |
MAZZEI L, BROLL V, CASALI L, et al. Multifunctional urea cocrystal with combined ureolysis and nitrification inhibiting capabilities for enhanced nitrogen management[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(15): 13369-13378. |
| [43] |
PARAKATAWELLA S, GOGOI D, DEKA P, et al. Mechanochemical synthesis of polymorphic urea ·adipic acid cocrystal as a sustained-release nitrogen source[J]. ChemSusChem, 2022. DOI:10.1002/cssc.202102445 |
| [44] |
ZHENG B, KABIRI S, ANDELKOVIC I B, et al. Mechanochemical synthesis of zinc borate for use as a dual-release B fertilizer[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(47): 15995-16004. |
| [45] |
SHARMA L, KIANI D, HONER K, et al. Mechanochemical synthesis of Ca-and Mg-double salt crystalline materials using insoluble alkaline earth metal bearing minerals[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(7): 6802-6812. |
| [46] |
BALTRUSAITIS J. Sustainable ammonia production[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(11): 9527-9527. |
| [47] |
MALINOWSKI P, BISKUPSKI A, GŁOWIŃSKI J. Preparation methods of calcium sulphate and urea adduct[J]. PJCT, 2007, 9(4): 111-114. DOI:10.2478/v10026-007-0102-z |
| [48] |
HONER K, KALFAOGLU E, PICO C, et al. Mechanosynthesis of magnesium and calcium salt-urea ionic cocrystal fertilizer materials for improved nitrogen management[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(10): 8546-8550. |
| [49] |
CASALI L, MAZZEI L, SHEMCHUK O, et al. Novel dual-action plant fertilizer and urease inhibitor: Urea ·catechol cocrystal, characterization and environmental reactivity[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(2): 2852-2859. |
| [50] |
HONER K, PICO C, BALTRUSAITIS J. Reactive mechanosynthesis of urea ionic cocrystal fertilizer materials from abundant low solubility magnesium-and calcium-containing minerals[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(4): 4680-4687. |
| [51] |
CASALI L, MAZZEI L, SHEMCHUK O, et al. Smart urea ionic co-crystals with enhanced urease inhibition activity for improved nitrogen cycle management[J]. Chemical Communications, 2018, 54(55): 7637-7640. DOI:10.1039/C8CC03777A |
| [52] |
JULIEN P A, GERMANN L S, TITI H M, et al. In situ monitoring of mechanochemical synthesis of calcium urea phosphate fertilizer cocrystal reveals highly effective water-based autocatalysis[J]. Chemical Science, 2020, 11(9): 2350-2355. DOI:10.1039/C9SC06224F |
| [53] |
BARČAUSKAITĖ K, BRAZIENĖ Z, AVIŽIENYTĖ D, et al. Mechanochemically synthesized gypsum and gypsum drywall waste cocrystals with urea for enhanced environmental sustainability fertilizers[J]. Journal of Environmental Chemical Engineering, 2020. DOI:10.1016/j.jece.2020.103965 |
| [54] |
SILVA M, BARCAUSKAITE K, DRAPANAUSKAITE D, et al. Relative humidity facilitated urea particle reaction with salicylic acid: A combined In situ spectroscopy and DFT study[J]. ACS Earth and Space Chemistry, 2020, 4(7): 1018-1028. DOI:10.1021/acsearthspacechem.0c00051 |
| [55] |
GONG L, LI T, CHEN F, et al. An inclusion complex of eugenol into β-cyclodextrin: Preparation, and physicochemical and antifungal characterization[J]. Food Chemistry, 2016, 196: 324-330. DOI:10.1016/j.foodchem.2015.09.052 |
| [56] |
PAVELA R. Essential oils as ecofriendly biopesticides? challenges and constraints[J]. Trends in Plant Science, 2016, 21(12): 1000-1007. DOI:10.1016/j.tplants.2016.10.005 |
| [57] |
CHAUDHARI A K, SINGH V K, KEDIA A, et al. Essential oils and their bioactive compounds as eco-friendly novel green pesticides for management of storage insect pests: Prospects and retrospects[J]. Environmental Science and Pollution Research, 2021, 28(15): 18918-18940. DOI:10.1007/s11356-021-12841-w |
| [58] |
BILIA A R, GUCCIONE C, ISACCHI B, et al. Essential oils loaded in nanosystems: A developing strategy for a successful therapeutic approach[J]. Evidence-Based Complementary and Alternative Medicine, 2014. DOI:10.1155/2014/651593 |
| [59] |
CARSON C F, HAMMER K A. Chemistry and bioactivity of essential oils[J]. Lipids and Essential Oils as Antimicrobial Agents, 2010, 203-238. |
| [60] |
KOUL O, WALIA S, DHALIWAL G. Essential oils as green pesticides: Potential and constraints[J]. Biopesticides International, 2008, 4(1): 63-84. |
| [61] |
FALLEH H, JEMAA M B, SAADA M. Essential oils: A promising eco-friendly food preservative[J]. Food Chemistry, 2020. DOI:10.1016/j.foodchem.2020.127268 |
| [62] |
SAROJ A, ORIYOMI O V, NAYAK A K, et al. Phytochemicals of plant-derived essential oils[M]//Natural Remedies for Pest, Disease and Weed Control. Amsterdam: Elsevier, 2020: 65-79
|
| [63] |
MAZZEO P P, CARRARO C, MONICA A, et al. Designing a palette of cocrystals based on essential oil constituents for agricultural applications[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(21): 17929-17940. |
| [64] |
MONTISCI F, MAZZEO P P, CARRARO C, et al. Dispensing essential oil components through cocrystallization: Sustainable and smart materials for food preservation and agricultural applications[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(26): 8388-8399. |
| [65] |
BIANCHI F, FORNARI F, RIBONI N, et al. Development of novel cocrystal-based active food packaging by a quality by design approach[J]. Food Chemistry, 2021, 347: 129051. DOI:10.1016/j.foodchem.2021.129051 |
| [66] |
BAKKALI F, AVERBECK S, AVERBECK D, et al. Biological effects of essential oils-A review[J]. Food and chemical toxicology, 2008, 46(2): 446-475. DOI:10.1016/j.fct.2007.09.106 |
| [67] |
SHARMA S, BARKAUSKAITE S, JAISWAL A K, et al. Essential oils as additives in active food packaging[J]. Food Chemistry, 2020. DOI:10.1016/j.foodchem.2020.128403 |
| [68] |
BOREL-SALADIN J M, TUROK I N. The green economy: Incremental change or transformation?[J]. Environmental Policy and Governance, 2013, 23(4): 209-220. DOI:10.1002/eet.1614 |
 2023, Vol. 40
2023, Vol. 40