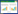硝基酚广泛应用于染料、增塑剂、农药、涂料、医药、炸药、木材或皮革防腐剂的合成[1-3],硝基及苯环的存在使其具有高毒、致癌性、难降解、生物富集等特点[3-4],进入生物体内易造成中枢系统和循环系统损伤[5]。目前硝基酚类化合物的降解主要有生物降解法和化学氧化法,但废水中高浓度的硝基酚会抑制菌种的生物活性[4, 6]。基于硫酸根自由基的高级氧化技术因其氧化能力强和适用范围广而受到人们的广泛关注。
过一硫酸盐(PMS)的氧化还原电位为1.82 V[7],可以被热[8]、碱[9]、光[8, 10]、过渡金属及其氧化物[11]、碳材料[7, 12]等活化生成具有更高氧化还原电势的羟基自由基(·OH)和硫酸根自由基(SO4·-)氧化有机物,也可以通过生成单线态氧1O2或通过表面电子转移形成络合物中间体的非自由基方式氧化有机物[13]。其中,含有Fe、Co和Cu等过渡金属氧化物的非均相催化反应具有氧化能力强、避免二次污染、方便回收利用等优势,引起人们的广泛关注。
在过渡金属离子活化PMS的均相反应中,Anipsitakis和Dionsious[14]比较了多种金属离子对PMS的活化能力,发现Co2+表现出最佳效果,因此,钴氧化物成为研究的热点。Anipsitakis等[15]首次将Co3O4用于PMS活化降解2, 4-二氯苯酚,表现出较好的催化效果。Co3O4所具有的Co2+和Co3+离子结构在活化PMS方面表现出优异的性能[16]。研究人员采取了改变Co3O4的尺寸形貌[17-18]、引入Mn或Fe等元素[19]或与碳材料等载体相结合[20]的方式来提高催化性能和降低钴离子的析出量。近年来,诸多研究发现可通过合成特定形貌的过渡金属氧化物从而改变反应的活性和选择性[21-23]。Mu等[24]制备了片状、棒状和立方块形Co3O4催化H2O2降解有机物,与后两者相比,片状Co3O4表现出更高的催化活性,研究认为催化活性的差异与所暴露晶面的特性有关。Saputra等[25]比较了立方块和球形Co3O4活化PMS降解苯酚,立方Co3O4表现出更好的催化性能。Sun等[26]概述了多种形貌Co3O4暴露的不同晶面,认为几何形状与晶面密切相关,共同影响着Co3O4在高级氧化领域的应用。硝基酚作为高毒且难降解的有机污染物,通过控制形貌制备具有高活性晶面的金属氧化物对于提高硝基酚的降解效果具有重要意义。目前,对比不同形貌Co3O4活化PMS用于降解硝基苯的研究仍未见报道。
本研究合成了不同形貌的Co3O4用于活化PMS,通过动力学实验比较了其对废水中不同种类硝基酚的降解速率差异,并分析阐述了其作用机理。
1 实验部分 1.1 试剂与仪器乙酸钴[Co(CH3COO)2·4H2O,AR]、硝酸钴[Co(NO3)2·6H2O,AR]、乙二醇(AR)、三苯基膦(AR)、碳酸钠(Na2CO3,AR)、氢氧化钠(NaOH, AR)、氨水(AR)、对硝基酚(4-NP,AR)、间硝基酚(3-NP,AR)、邻硝基酚(2-NP,AR)、2, 4-二硝基酚钠(2, 4-DNP,AR)、乙腈(GC)、过一硫酸盐(2KHSO5·KHSO4·K2SO4,PMS,AR)、甲醇(MeOH,AR)、叔丁醇(TBA,AR)、糠醇(FFA,AR)、5, 5-二甲基-1-吡咯啉-N-氧化物(DMPO,97%)和2, 2, 6, 6-四甲基-4-哌啶酮(TEMP, >98%)均购自上海阿拉丁生化科技股份有限公司。
1.2 实验方法 1.2.1 Co3O4的合成八面体Co3O4的合成按照Zhou等[27]的方法进行:浓度0.40 mmol·L-1的三苯基膦和1.7 mmol·L-1 Co(NO3)2·6H2O溶解在120 mL去离子水中。向上述溶液中加入2.0 mol·L-1 NaOH调节pH=7.6,将混合溶液转移至150 mL水热釜,180 ℃反应24 h,冷却至室温后,离心洗涤数次,将所得产物在60 ℃真空干燥12 h。
立方体Co3O4的制备按Dong等[28]的方法进行:将1.0 g Co (CH3COO)2·4H2O溶于50.0 mL水中,并在搅拌过程中加入5 mL 25%的氨水,混合搅拌10 min后形成均匀混合液,并将其转移到100 mL水热釜中,250 ℃下反应3 h,冷却至室温后将所得黑色固体离心洗涤,60 ℃真空干燥12 h。
棒状Co3O4的合成参考Fan[29]的方法进行:将5.0 g Co (CH3COO)2·4H2O溶于60 mL乙二醇溶液,将200 mL 0.2 mol·L-1 Na2CO3溶液在氮气流中逐滴滴入剧烈搅拌的乙二醇溶液中,冷凝回流1 h后冷却至室温,离心洗涤后将所得固体于50 ℃真空干燥并在空气氛围下450 ℃焙烧4 h。
1.2.2 硝基酚的降解室温下,将0.36 mmol·L-1硝基酚,4.0 mmol·L-1 PMS,1.0 g·L-1 Co3O4及一定量的自由基捕获剂(如需要),调节pH值后混合加入在250 mL锥形瓶内磁力搅拌。反应过程中使用0.5 mol·L-1的NaOH调节pH值,所有反应均在pH值为8.0条件下进行。一定时间内进行取样,样品采用0.22 μm滤膜过滤后使用HPLC测定其浓度。
降解率计算方法为:
| $ \text { 降解率 }=\frac{\left(C_{0}-C\right)}{C_{0}} \times 100 \% $ | (1) |
表观反应速率常数k计算方法为:
| $ \ln \left(\frac{C}{C_{0}}\right)=-k t $ | (2) |
式(1)和(2)中:C0为硝基酚初始浓度;mmol·L-1;C为取样所测得的浓度,mmol·L-1。
1.2.3 分析方法使用高效液相色谱(HPLC, 岛津, LC-20)检测水体硝基酚含量,使用C18色谱柱(250 mm×4.6 mm, 5 μm),柱温30 ℃,流速0.6 mL·min-1,流动相为V(乙腈):V(水)=6:4,进样量10 μL,使用电感耦合等离子体质谱(ICP, 赛默飞, iCAP Qc7400)对体系中析出的Co2+进行检测;总有机碳测定仪(TOC, 日本岛津, TOC-L)测定反应前后总有机碳变化;使用电子自旋共振顺磁波谱仪(ESR,德国布鲁克,JEOL FA200)捕获自由基进行检验。
使用X射线衍射(XRD, 德国布鲁克,D8 ADVANCE)对所制备Co3O4的晶型结构进行表征,Cu_Kα辐射源,测试电压为40 kV,扫描范围15°~70°。扫描电子显微镜(SEM,日本日立,S-4800)和透射电子显微镜(TEM,日本电子,JEM-2100F)对Co3O4形貌进行表征。N2吸附脱附曲线测定比表面积(BET,美国麦克莫瑞提克,ASAP 2020)。
2 结果与讨论 2.1 晶体结构分析不同制备条件下的Co3O4样品的XRD谱图如图 1所示,3种方法所制备的Co3O4拥有相似的衍射峰,在2θ为19.0°、31.3°、36.9°、38.5°、44.8°、55.7°、59.4°和65.2°处的衍射峰分别对应(111)、(220)、(311)、(222)、(400)、(422)、(511)和(440)晶面,与Co3O4的标准XRD图谱(JCDPS 42-1467)一致,表明所制备的产物为Co3O4[24]。3种Co3O4对应的XRD图谱峰形尖锐且无杂质峰,说明制备的Co3O4均拥有较高的结晶度和纯度[25]。

|
| 图 1 不同方法制备的XRD图谱 Fig.1 XRD pattern of the samples with different synthesizing methods |
| |
不同形貌Co3O4的微观形貌如图 2所示。3种制备方法所得Co3O4表现出完全不同的形貌特征。

|
| 图 2 a) 和b)八面体、c)和d)立方块和e)和f)棒状Co3O4的SEM和TEM图谱 Fig.2 SEM and TEM images of a) and b) octahedral Co3O4, c) and d) cubic Co3O4, e) and f) rodlike Co3O4 |
| |
由图 2a)及图 2b)可以看出,八面体Co3O4的颗粒尺寸为1~2 μm。图 2c)及图 2d)表明,立方块Co3O4呈单分散立方块分布,尺寸约为0.1~0.4 μm。图 2e)和图 2f)表明,棒状Co3O4直径约10 nm, 长约50~80 nm,棒状Co3O4的粒径较小,在反应体系中有更好的分散性。采用BET法测试了八面体、立方块和棒状Co3O4的比表面积依次为18.4、19.6和66.4 m2·g-1。
2.3 不同形貌Co3O4对硝基酚的降解效果实验对不同形貌Co3O4催化PMS分别氧化四种硝基酚的能力进行了比较。在未有Co3O4活化的条件下,PMS自身几乎不与硝基酚反应。此外,实验表明不同形貌Co3O4在5 h内对硝基酚的吸附量可以忽略不计,因此可以排除反应中吸附对结果的影响。如图 3a)所示,八面体Co3O4活化PMS反应5 h后对4种硝基酚降解的能力为:3-NP (93.4%)>4-NP (83.8%)>2-NP (80.6%)>2, 4-DNP (56.5%),反应速率常数分别为0.029 7、0.018 6、0.017 2和0.009 0 g·m-2·h-1。图 3b)表明,立方块状Co3O4反应5 h后对4种硝基酚降解的反应活性依次为:3-NP (98.3%)>4-NP (86.0%)>2-NP (81.6%)>2, 4-DNP (61.4%),反应速率分别为0.059 7、0.020 1、0.017 9和0.010 1 g·m-2·h-1。图 3c)显示棒状Co3O4作为催化剂活化PMS在2 h内,能够将4种硝基酚几乎降解完全,反应速率常数为0.089 5、0.074 6、0.047 7和0.042 8 g·m-2·h-1。实验结果表明相比于八面体Co3O4和立方块Co3O4,棒状活化PMS对硝基酚具有更强的氧化能力。硝基(—NO2)在苯环上相对—OH的位置差异使其对—OH发生差异性的吸电子作用,导致各Co3O4氧化体系对不同硝基酚产生降解能力的差异[30]。

|
| 图 3 a) 八面体Co3O4, b)立方块Co3O4; c)棒状Co3O4在Co3O4/PMS体系中对硝基酚的去除效果及其d)反应速率k Fig.3 Removal efficiency of nitrophenols for a) octahedral Co3O4, b) cubic Co3O4, c) rodlike Co3O4 and d) the rate constant k |
| |
为排除粒径和比表面积差异对评价催化剂性能的影响,通过引入比表面积对速率常数归一化处理后[图 3d)][31-32]可以看出,立方块Co3O4的催化能力略高于八面体,而棒状Co3O4则明显大于其余2种Co3O4,为八面体Co3O4的2.4~4.0倍,这可能是由于不同形貌Co3O4暴露的晶面不同所导致的。通过改变晶面的生长速度可以控制Co3O4形貌的合成,(110)面为棒状Co3O4主要晶面,(100)面为立方块Co3O4的主要晶面,(111)面为八面体的主要晶面[23-25];不同晶面上原子排列差异使其表现出不同的电子转移能力进而影响了Co3O4的催化活性[25-26]。
此外,对硝基酚降解前后溶液进行TOC测试,结果如表 1所示。随着硝基酚的降解,体系中TOC含量有明显下降,表明随反应进行,硝基酚在PMS氧化下,被逐步矿化为CO2、H2O等小分子无机物。通过对比反应后体系中TOC的剩余量的不同分析其催化性能的差异,可以得出与动力学实验相类似的结论:棒状Co3O4>立方块Co3O4>八面体Co3O4。Hu[33]采用0.1 g·L-1的Fe3O4在微波作用下活化过二硫酸盐降解20 mg·L-1的4-NP,28 min后4-NP的去除率达98.2%,去除TOC为53.2%;Li等[34]使用30 g·L-1 CuFe2O4活化8 mmol·L-1的过二硫酸盐处理50 mg·L-1的4-NP,去除率为89%时,TOC去除率为81%。实验中八面体、立方块、棒状3种形貌Co3O4对4-NP的TOC去除率依次为60.5%、70.03%和85.03%,表明所合成的Co3O4活化PMS后表现出较强的氧化性能,与动力学反应结果一致。
| 硝基酚种类 | Co3O4种类 | TOC/(mg·L-1) |
| 4-NP | 反应前 | 25.203 |
| 八面体Co3O4 | 9.952 | |
| 立方块Co3O4 | 7.553 | |
| 棒状Co3O4 | 3.722 | |
| 2-NP | 反应前 | 25.361 |
| 八面体Co3O4 | 12.772 | |
| 立方块Co3O4 | 8.291 | |
| 棒状Co3O4 | 5.854 | |
| 3-NP | 反应前 | 25.433 |
| 八面体Co3O4 | 5.458 | |
| 立方块Co3O4 | 2.356 | |
| 棒状Co3O4 | 1.558 | |
| 2, 4-DNP | 反应前 | 25.023 |
| 八面体Co3O4 | 8.871 | |
| 立方块Co3O4 | 8.070 | |
| 棒状Co3O4 | 6.764 |
反应5 h后,使用ICP测试八面体、立方块和棒状Co3O4在降解4-NP后Co2+的析出量依次为0.032、0.029和0.022 mg·L-1,这表明在pH值为8.0的弱碱性环境下,有效避免了Co2+的析出,有利于的Co3O4重复利用。Co2+同样能够活化PMS。如图 4所示,分别取5 h后3种Co3O4降解4-NP的上清液,与PMS和4-NP混合,未检测到硝基酚含量的明显降低,说明微量的Co2+对PMS活化能力较弱,排除均相反应降解硝基酚的可能。

|
| 图 4 体系析出的Co2+对4-NP的降解情况 Fig.4 Removal efficiency of 4-NP for Co2+ leached from system |
| |
实验采用甲醇(MeOH)、叔丁醇(TBA)、糠醇(FFA)、对苯醌(p-BQ)自由基捕获剂,对Co3O4/PMS体系降解4-NP的活性氧化物种进行分析,结果如图 5所示。向体系中加入2.0 mol·L-1甲醇作为·OH和SO4·-自由基捕获剂(k(·OH, MeOH)=2.5×107 L·mol-1·s-1;k(SO4·-,MeOH)=9.7×108 L·mol-1·s-1)[36-37],4-NP降解效果受到明显抑制,说明·OH或SO4·-参与了硝基酚的降解。加入2.0 mmol·L-1叔丁醇(TBA)捕获体系中的·OH [k(·OH, TBA)=(3.8~7.6)×108 L·mol-1·s-1][36, 38]。由图 5a)~图 5c)可以看出,八面体、立方块和棒状Co3O4/PMS体系在加入TBA后,4-NP的降解效果分别从83.8%降低到55.0%,86.0%降低到57.8%,99.7%降低到67.3%.尽管4-NP的降解受到一定程度的抑制,但仍保持较高的降解率,表明·OH和SO4·-都参与到硝基酚的降解,其中SO4·-起更主要作用。同时,有文献报道1O2和超氧自由基(O2·-)同样是PMS用于降解有机物过程中的常见活性物种。实验中,用5 mmol·L-1糠醇(FFA)作为1O2捕获剂(k(1O2, FFA)=1.2×108 L·mol-1·s-1)[39],由图 5a)~图 5d)可以看出,FFA的加入同样在一定程度上抑制了4-NP的降解。5 mmol·L-1对苯醌(p-BQ)作为O2·-捕获剂加入反应体系[40]。由图 5d)可以看出,反应速率受到的影响较小,证实体系中O2·-未参与到硝苯酚的降解。不同于一些文献报道的1O2产生自超氧自由基HO2·/O2·-的重组[9, 41-42],1O2可能是来自催化剂表面的氧空位[43-44]。

|
| 图 5 Co3O4/PMS体系中不同抑制剂对4-NP降解效果的影响: a)八面体Co3O4、b)立方块Co3O4; c)棒状Co3O4和d)反应速率k Fig.5 Inhibition effects of different scavengers on the removal of 4-NP in Co3O4/PMS system with a) octahedral Co3O4, b) cubic Co3O4, c) rodlike Co3O4 and d) the corresponding rate constant k |
| |
为进一步探究硝基酚在Co3O4/PMS体系降解中起作用的活性物种,使用DMPO作为·OH和SO4·-的捕获剂,TEMP作为1O2的自由基捕获剂,进行ESR测试,测得自由基信号如图 6所示。

|
| 图 6 不同形貌Co3O4在Co3O4/PMS体系的ESR谱图 Fig.6 ESR spectrum of Co3O4 with different morphologies in Co3O4/PMS system |
| |
在DMPO的作用下,不同形貌Co3O4激活PMS时,均可以观察到DMPO-OH (4重峰, 1:2:2:1)和DMPO-SO4(6重峰,1:1:1:1:1:1)信号[45-46][图 6a)],表明·OH和SO4·-同时存在与反应体系中。DMPO也可以与硝基酚降解过程中产生的小分子碳中心自由基形成DMPO-R[43, 47],进一步证实硝基酚在降解过程中经历了被转化为其他中间产物再进一步被矿化。图 6b)中强烈的三重峰信号(1:1:1)来自TEMP与1O2结合产生的TEMP-1O2,这进一步证实反应体系中1O2产生[48]。Co3O4的加入使TEMP-1O2信号显著增强,表明Co3O4的存在促进了1O2的生成。此外,棒状Co3O4在激活PMS过程中所产生的信号最为明显,表明棒状Co3O4能够更迅速促进活性氧化物种的产生,这与动力学实验中棒状Co3O4激活PMS降解速率最快的现象相吻合。实验过程中未检测到DMPO-O2信号,证明O2·-未参与到硝基酚的降解。因此,自由基淬灭实验和ESR实验结果共同证实了·OH、SO4·-和1O2是反应体系中的主要活性物种。
2.6 反应机制分析在Co3O4/PMS体系中,表面的L酸位点通过电子转移使PMS中的O—O键断裂,迅速产生SO4·-[7, 49],SO4·-又可以进一步与H2O或HSO5-作用生成·OH,二者共同作用,增强对硝基酚的降解[式(3)~式(7)][50]。
| $ {{\rm{C}}{{\rm{o}}^{2 + }} + {\rm{HSO}}_5^ - \to {\rm{C}}{{\rm{o}}^{3 + }} + {\rm{SO}}_4^{ \cdot - } + {\rm{O}}{{\rm{H}}^ - }} $ | (3) |
| $ {{\rm{C}}{{\rm{o}}^{2 + }} + {\rm{HSO}}_5^ - \to {\rm{C}}{{\rm{o}}^{3 + }} + {\rm{SO}}_4^{2 - } + \cdot {\rm{OH}}} $ | (4) |
| $ {{\rm{C}}{{\rm{o}}^{3 + }} + {\rm{HSO}}_5^ - \to {\rm{C}}{{\rm{o}}^{2 + }} + {\rm{SO}}_5^{ \cdot - } + {{\rm{H}}^ + }} $ | (5) |
| $ {\rm{SO}}_4^{ \cdot - } + {{\rm{H}}_2}{\rm{O}} \to \cdot {\rm{OH}} + {\rm{SO}}_4^{2 - } + {{\rm{H}}^ + } $ | (6) |
| $ ·{\rm{OH}} + {\rm{SO}}_4^{ \cdot - } + {\rm{NPs}} \to \cdots \to {\rm{C}}{{\rm{O}}_2} + {{\rm{H}}_2}{\rm{O}} + {\rm{SO}}_4^{2 - } $ | (7) |
另一方面,1O2的产生同样促进了反应的进行。过渡金属氧化物中Co-O-Co在反应过程中产生氧空位(VO), 促进活性氧化物质*O的产生,并进一步转化为1O2[51]加速了硝基酚的降解[式(8)~式(10)]。
| $ {{\rm{HSO}}_5^ - { + ^*}{\rm{O}} \to {\rm{HSO}}_4^ - { + ^1}{{\rm{O}}_2}} $ | (8) |
| $ {{\rm{C}}{{\rm{o}}^{3 + }} + {{\rm{O}}^{2 - }} \to {\rm{C}}{{\rm{o}}^{3 + }} + {{\rm{V}}_0}{ + ^*}{\rm{O}} \to {\rm{C}}{{\rm{o}}^{2 + }}{ + ^*}{\rm{O}}} $ | (9) |
| $ {{\rm{HSO}}_5^ - + {\rm{SO}}_5^{2 - } \to {\rm{HSO}}_4^ - + {\rm{SO}}_4^{2 - }{ + ^1}{{\rm{O}}_2}} $ | (10) |
综上,Co3O4/PS体系氧化4-NP涉及以·OH和SO4·-为主的自由基反应,也涉及生成1O2的非自由基反应。通过计算自由基抑制剂实验下表观反应速率与空白条件下的k值之比,可以发现与FFA捕获1O2相比,MeOH捕获·OH和SO4·-条件下反应被抑制的程度较大,自由基在反应过程中起到重要作用,且氧化途径与形貌差异有关,可以通过对Co3O4的形貌进行调控改变反应途径,从而改善其催化性能;而Co3O4表面氧空位的出现有利于界面上的电子转移[52-53],基于的1O2非自由基过程也促进了4-NP的降解。
2.7 重复性实验将反应后3种形貌的Co3O4回收后进行重复试验,结果如图 7所示。

|
| 图 7 八面体Co3O4、立方块Co3O4和棒状Co3O4降解4-NP循环试验 Fig.7 Reusability of octahedral Co3O4, cubic Co3O4 and rodlike Co3O4 in the removal of 4-NP |
| |
3种形貌的Co3O4在多次循环使后仍能表现出较好的催化性能,经过5次循环使用后,4-NP的降解率仍能保持在63%以上,表明3种形貌的催化剂稳定性较好。
3 结论1) 实验成功制备了八面体、立方块和棒状3种不同形貌的Co3O4。3种Co3O4在Co3O4/PMS体系降解废水中的硝基酚时现出不同的催化活性:棒状Co3O4>立方块Co3O4>八面体Co3O4;不同种类硝基酚的降解难度按照2, 4-DNP,2-NP,4-NP和3-NP的顺序依次降低。
2) 3种Co3O4在pH值为8.0下活化PMS析出的Co2+含量较低,且均表现出良好的循环利用性。
3) Co3O4/PMS体系降解硝基酚通过自由基机制和非自由基机制共同作用,起作用的活性氧化物种主要为·OH、SO4·-和1O2。
| [1] |
Sponza D T, Kuscu Ö S. Relationships between acute toxicities of para nitrophenol (p-NP) and nitrobenzene (NB) to Daphnia magna and Photobacterium phosphoreum:Physicochemical properties and metabolites under anaerobic/aerobic sequentials[J]. Journal of Hazardous Materials, 2011, 185(2/3): 1187-1197. |
| [2] |
Li J, Liu Q, Ji Q, et al. Degradation of p-nitrophenol (PNP) in aqueous solution by Fe0-PM-PS system through response surface methodology (RSM)[J]. Applied Catalysis B:Environmental, 2017, 200: 633-646. DOI:10.1016/j.apcatb.2016.07.026 |
| [3] |
She Z, Gao M, Jin C, et al. Toxicity and biodegradation of 2, 4-dinitrophenol and 3-nitrophenol in anaerobic systems[J]. Process Biochemistry, 2005, 40(9): 3017-3024. DOI:10.1016/j.procbio.2005.02.007 |
| [4] |
Xiong Z, Zhang H, Zhang W, et al. Removal of nitrophenols and their derivatives by chemical redox:A review[J]. Chemical Engineering Journal, 2019, 359: 13-31. DOI:10.1016/j.cej.2018.11.111 |
| [5] |
Silva E F, Varela Junior A S, Cardoso T F, et al. Reproductive toxicology of 2, 4 dinitrophenol in boar sperm[J]. Toxicology in Vitro, 2016, 35: 31-35. DOI:10.1016/j.tiv.2016.05.002 |
| [6] |
Arora P K, Srivastava A, Singh V P. Bacterial degradation of nitrophenols and their derivatives[J]. Journal of Hazardous Materials, 2014, 266: 42-59. DOI:10.1016/j.jhazmat.2013.12.011 |
| [7] |
Wang J, Wang S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants[J]. Chemical Engineering Journal, 2018, 334: 1502-1517. DOI:10.1016/j.cej.2017.11.059 |
| [8] |
Yang S, Wang P, Yang X, et al. Degradation efficiencies of azo dye Acid Orange 7 by the interaction of heat, UV and anions with common oxidants:Persulfate, peroxymonosulfate and hydrogen peroxide[J]. Journal of Hazardous Materials, 2010, 179(1/2/3): 552-558. |
| [9] |
Qi C, Liu X, Ma J, et al. Activation of peroxymonosulfate by base:Implications for the degradation of organic pollutants[J]. Chemosphere, 2016, 151: 280-288. DOI:10.1016/j.chemosphere.2016.02.089 |
| [10] |
Liu X, Zhang T, Zhou Y, et al. Degradation of atenolol by UV/peroxymonosulfate:Kinetics, effect of operational parameters and mechanism[J]. Chemosphere, 2013, 93(11): 2717-2724. DOI:10.1016/j.chemosphere.2013.08.090 |
| [11] |
Hu P, Long M. Cobalt-Catalyzed sulfate radical-based advanced oxidation:A review on heterogeneous catalysts and applications[J]. Applied Catalysis B:Environmental, 2016, 181: 103-117. DOI:10.1016/j.apcatb.2015.07.024 |
| [12] |
Sun H, Kwan C, Suvorova A, et al. Catalytic oxidation of organic pollutants on pristine and surface nitrogen-modified carbon nanotubes with sulfate radicals[J]. Applied Catalysis B:Environmental, 2014, 154/155: 134-141. DOI:10.1016/j.apcatb.2014.02.012 |
| [13] |
Duan X, Ao Z, Zhou L, et al. Occurrence of radical and nonradical pathways from carbocatalysts for aqueous and nonaqueous catalytic oxidation[J]. Applied Catalysis B:Environmental, 2016, 188: 98-105. DOI:10.1016/j.apcatb.2016.01.059 |
| [14] |
Anipsitakis G P, Dionysiou D D. Radical generation by the interaction of transition metals with common oxidants[J]. Environmental Science & Technology, 2004, 38(13): 3705-3712. |
| [15] |
Anipsitakis G P, Stathatos E, Dionysiou D D. Heterogeneous activation of oxone using Co3O4[J]. Journal of Physical Chemistry B, 2005, 109(27): 13052-13055. DOI:10.1021/jp052166y |
| [16] |
Saputra E, Muhammad S, Sun H, et al. A comparative study of spinel structured Mn3O4, Co3O4 and Fe3O4 nanoparticles in catalytic oxidation of phenolic contaminants in aqueous solutions[J]. Journal of Colloid and Interface Science, 2013, 407: 467-473. DOI:10.1016/j.jcis.2013.06.061 |
| [17] |
Chen X, Chen J, Qiao X, et al. Performance of nano-Co3O4/peroxymonosulfate system:Kinetics and mechanism study using Acid Orange 7 as a model compound[J]. Applied Catalysis B:Environmental, 2008, 80(1/2): 116-121. |
| [18] |
Deng J, Feng S, Zhang K, et al. Heterogeneous activation of peroxymonosulfate using ordered mesoporous Co3O4 for the degradation of chloramphenicol at neutral pH[J]. Chemical Engineering Journal, 2017, 308: 505-515. DOI:10.1016/j.cej.2016.09.075 |
| [19] |
Liang H, Sun H, Patel A, et al. Excellent performance of mesoporous Co3O4/MnO2 nanoparticles in heterogeneous activation of peroxymonosulfate for phenol degradation in aqueous solutions[J]. Applied Catalysis B:Environmental, 2012, 127: 330-335. DOI:10.1016/j.apcatb.2012.09.001 |
| [20] |
Al Nafiey A, Addad A, Sieber B, et al. Reduced graphene oxide decorated with Co3O4 nanoparticles (rGO-Co3O4) nanocomposite:A reusable catalyst for highly efficient reduction of 4-nitrophenol, and Cr(VI) and dye removal from aqueous solutions[J]. Chemical Engineering Journal, 2017, 322: 375-384. DOI:10.1016/j.cej.2017.04.039 |
| [21] |
Pal J, Pal T. Faceted metal and metal oxide nanoparticles:Design, fabrication and catalysis[J]. Nanoscale, 2015, 7(34): 14159-14190. DOI:10.1039/C5NR03395K |
| [22] |
Li Y, Shen W. Morphology-Dependent nanocatalysts:Rod-Shaped oxides[J]. Chemical Society Reviews, 2014, 43(5): 1543-1574. DOI:10.1039/C3CS60296F |
| [23] |
Cao S, Tao F, Tang Y, et al. Size- and shape-dependent catalytic performances of oxidation and reduction reactions on nanocatalysts[J]. Chemical Society Reviews, 2016, 45(17): 4747-4765. DOI:10.1039/C6CS00094K |
| [24] |
Mu J, Zhang L, Zhao G, et al. The crystal plane effect on the peroxidase-like catalytic properties of Co3O4 nanomaterials[J]. Physical Chemistry Chemical Physics, 2014, 16(29): 15709-15716. DOI:10.1039/c4cp01326c |
| [25] |
Saputra E, Duan X, Pinem J A, et al. Shape-Controlled Co3O4 catalysts for advanced oxidation of phenolic contaminants in aqueous solutions[J]. Separation and Purification Technology, 2017, 186: 213-217. DOI:10.1016/j.seppur.2017.02.057 |
| [26] |
Sun H, Ang H M, Tadé M O, et al. Co3O4 nanocrystals with predominantly exposed facets:Synthesis, environmental and energy applications[J]. Journal of Materials Chemistry A, 2013, 1(46): 14427-14442. DOI:10.1039/c3ta12960h |
| [27] |
Zhou H, Lü B, Wu D, et al. Synthesis and properties of octahedral Co3O4 single-crystalline nanoparticles enclosed by (111) facets[J]. CrystEngComm, 2013, 15(41): 8337-8344. DOI:10.1039/c3ce41419a |
| [28] |
Dong Y, He K, Yin L, et al. A facile route to controlled synthesis of Co3O4 nanoparticles and their environmental catalytic properties[J]. Nano Technology, 2007. DOI:10.1088/0957-4484/18/43/435602 |
| [29] |
Fan Z, Fang W, Zhang Z, et al. Highly active rod-like Co3O4 catalyst for the formaldehyde oxidation reaction[J]. Catalysis Communications, 2018, 103: 10-14. DOI:10.1016/j.catcom.2017.09.003 |
| [30] |
di Paola A, Augugliaro V, Palmisano L, et al. Heterogeneous photocatalytic degradation of nitrophenols[J]. Journal of Photochemistry and Photobiology A:Chemistry, 2003, 155(1/2/3): 207-214. |
| [31] |
Li C, Sun Y, Djerdj I, et al. Shape-Controlled CeO2 nanoparticles:Stability and activity in the catalyzed HCl oxidation reaction[J]. ACS Catalysis, 2017, 7(10): 6453-6463. DOI:10.1021/acscatal.7b01618 |
| [32] |
Du X, Zhang Y, Si F, et al. Persulfate non-radical activation by nano-CuO for efficient removal of chlorinated organic compounds:Reduced graphene oxide-assisted and CuO (001) facet-dependent[J]. Chemical Engineering Journal, 2019, 356: 178-189. DOI:10.1016/j.cej.2018.08.216 |
| [33] |
Hu L, Wang P, Liu G, et al. Catalytic degradation of p-nitrophenol by magnetically recoverable Fe3O4 as a persulfate activator under microwave irradiation[J]. Chemosphere, 2020. |
| [34] |
Li J, Ren Y, Ji F, et al. Heterogeneous catalytic oxidation for the degradation of p-nitrophenol in aqueous solution by persulfate activated with CuFe2O4 magnetic nano-particles[J]. Chemical Engineering Journal, 2017, 324: 63-73. DOI:10.1016/j.cej.2017.04.104 |
| [35] |
Xiao R, Luo Z, Wei Z, et al. Activation of peroxymonosulfate/persulfate by nanomaterials for sulfate radical-based advanced oxidation technologies[J]. Current Opinion in Chemical Engineering, 2018, 19: 51-58. DOI:10.1016/j.coche.2017.12.005 |
| [36] |
Buxton G V, Mulazzani Q G, Ross A B. Critical review of rate constants for reactions of transients from metal ions and metal complexes in aqueous solution[J]. Journal of Physical and Chemical Reference Data, 1995, 24(3): 1055-1349. DOI:10.1063/1.555966 |
| [37] |
Zhang T, Zhu H, Croué J P. Production of sulfate radical from peroxymonosulfate induced by a magnetically separable CuFe2O4 spinel in water:Efficiency, stability, and mechanism[J]. Environmental Science & Technology, 2013, 47(6): 2784-2791. |
| [38] |
Zhao Y, An H, Feng J, et al. Impact of crystal types of AgFeO2 nanoparticles on the peroxymonosulfate activation in the water[J]. Environmental Science & Technology, 2019, 53(8): 4500-4510. |
| [39] |
Zhu S, Li X, Kang J, et al. Persulfate activation on crystallographic manganese oxides:Mechanism of singlet oxygen evolution for nonradical selective degradation of aqueous contaminants[J]. Environmental Science & Technology, 2019, 53(1): 307-315. |
| [40] |
Wang Y, Cao D, Zhao X. Heterogeneous degradation of refractory pollutants by peroxymonosulfate activated by CoOx-doped ordered mesoporous carbon[J]. Chemical Engineering Journal, 2017, 328: 1112-1121. DOI:10.1016/j.cej.2017.07.042 |
| [41] |
Liu Y, Guo H, Zhang Y, et al. Heterogeneous activation of peroxymonosulfate by sillenite Bi25FeO40:Singlet oxygen generation and degradation for aquatic levofloxacin[J]. Chemical Engineering Journal, 2018, 343: 128-137. |
| [42] |
Yang S, Wu P, Liu J, et al. Efficient removal of bisphenol a by superoxide radical and singlet oxygen generated from peroxymonosulfate activated with Fe0-montmorillonite[J]. Chemical Engineering Journal, 2018, 350: 484-495. DOI:10.1016/j.cej.2018.04.175 |
| [43] |
Chen Z, Bi S, Zhao G, et al. Enhanced degradation of triclosan by cobalt manganese spinel-type oxide activated peroxymonosulfate oxidation process via sulfate radicals and singlet oxygen:Mechanisms and intermediates identification[J]. Science of the Total Environment, 2020, 711: 134715. DOI:10.1016/j.scitotenv.2019.134715 |
| [44] |
Tian X, Gao P, Nie Y, et al. A novel singlet oxygen involved peroxymonosulfate activation mechanism for degradation of ofloxacin and phenol in water[J]. Chemical Communications, 2017, 53(49): 6589-6592. DOI:10.1039/C7CC02820B |
| [45] |
Wei Z, Villamena F A, Weavers L K. Kinetics and mechanism of ultrasonic activation of persulfate:An in situ EPR spin trapping study[J]. Environmental Science & Technology, 2017, 51(6): 3410-3417. |
| [46] |
Lim J, Yang Y, Hoffmann M R. Activation of peroxymonosulfate by oxygen vacancies-enriched cobalt-doped black TiO2 nanotubes for the removal of organic pollutants[J]. Environmental Science & Technology, 2019, 53(12): 6972-6980. |
| [47] |
Fang G, Chen X, Wu W, et al. Mechanisms of interaction between persulfate and soil constituents:Activation, free radical formation, conversion, and identification[J]. Environmental Science & Technology, 2018, 52(24): 14352-14361. |
| [48] |
Zhou Y, Jiang J, Gao Y, et al. Activation of peroxymonosulfate by benzoquinone:A novel nonradical oxidation process[J]. Environmental Science & Technology, 2015, 49(21): 12941-12950. |
| [49] |
Wang Y, Zhou L, Duan X, et al. Photochemical degradation of phenol solutions on Co3O4 nanorods with sulfate radicals[J]. Catalysis Today, 2015, 258: 576-584. DOI:10.1016/j.cattod.2014.12.020 |
| [50] |
Jiang M, Zhang Q, Ji Y, et al. Transformation of antimicrobial agent sulfamethazine by peroxymonosulfate:Radical vs. nonradical mechanisms[J]. Science of the Total Environment, 2018, 636: 864-871. DOI:10.1016/j.scitotenv.2018.04.321 |
| [51] |
Chen Z, Kronawitter C X, Koel B E. Facet-Dependent activity and stability of Co3O4 nanocrystals towards the oxygen evolution reaction[J]. Physical Chemistry Chemical Physics, 2015, 17(43): 29387-29393. DOI:10.1039/C5CP02876K |
| [52] |
Lu S, Wang G, Chen S, et al. Heterogeneous activation of peroxymonosulfate by LaCo1-xCuxO3 perovskites for degradation of organic pollutants[J]. Journal of Hazardous Materials, 2018, 353: 401-409. |
| [53] |
Gao P, Tian X, Nie Y, et al. Promoted peroxymonosulfate activation into singlet oxygen over perovskite for ofloxacin degradation by controlling the oxygen defect concentration[J]. Chemical Engineering Journal, 2019, 359: 828-839. DOI:10.1016/j.cej.2018.11.184 |
 2020, Vol. 37
2020, Vol. 37