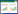我国是世界上最大的煤炭生产国与消费国,燃煤产生的SO2和NOx造成的污染严重影响了人类的身体健康和居住环境[1]。烟气脱硫是目前世界上控制酸雨和SO2污染的有效和主要的技术手段。现阶段应用最为广泛的脱硫方法有湿式石灰石/石膏法、氨法烟气脱硫、循环流化床烟气脱硫、海水脱硫技术以及电子束照射法脱硫技术。对于氮氧化物的控制,目前使用最广泛的是以V2O5为催化剂,NH3为还原剂将NOx还原成对环境无害的N2和H2O(SCR)。但是SCR技术消耗NH3和催化剂,存在着催化剂费用高及NH3的泄露等缺点。在众多的烟气脱硫脱硝方法中,干法烟气脱硫脱硝技术具有无污水和废酸排出、二次污染少、设备腐蚀程度轻,烟气在净化过程中无明显降温等优点,已经受到了业界的普遍关注[2]。采用吸附的方法脱除烟气中的酸性气体,由于吸附材料表面积大、去除效率高、具备选择性,可再生能力以及设计简单和可操作性好,引起了研究者的普遍兴趣。根据吸附材料的不同又可以分为活性炭吸附法和活性焦吸附法,其脱硫脱硝原理基本相同。
活性碳吸附方法具有比表面积大、内部孔隙结构发达、丰富的表面物种等优点,同时还具有一定的负载和还原性能,因此被广泛地应用于治理SO2和NOx等烟气污染物[3-5]。从废弃材料得到的活性炭经过碳化和化学处理后是一种适合处理烟气的吸附剂。
改性活性炭脱硫脱硝方法是以活性炭为载体,采用洗涤、浸渍活性组分和微波处理等手段对活性炭进行特殊处理,改变或改善活性炭的表面官能团、活性位以提高其脱硫脱硝活性的方法[2, 6],具体的改性方法以及在脱硫脱硝中的作用如表 1所示。
| 序号 | 活性炭改性方式 | 在烟气脱硫脱硝中的作用 |
| 1 | 负载金属离子或金属氧化物 | 在烟气脱硫中显示出较好的催化活性,选择性和稳定性;促进NO氧化为NO2,从而提高吸附效果。 |
| 2 | 化学浸渍 | 可以提高脱硫精度。 |
| 3 | 高温热处理 | 使活性炭表面酸性集团、表面积和孔结构变化,提高脱硫活性。 |
| 4 | 微波改性 | 可以扩张活性炭的比表面积,通过加热及诱发作用有效提高脱硫脱硝性能。 |
| 5 | 稀土改性 | 可以同时脱硫脱硝,降低反应温度。 |
活性炭在脱硫过程中既是吸附剂也是SO2氧化过程的催化剂,其反应机理为如图 1所示[6]。

|
| 图 1 活性炭表面脱硫机理 Fig.1 Defulfurization mechanism of surface on active carbon |
| |
总的过程可以用反应方程式表示为:
| $ 2{\rm{S}}{{\rm{O}}_2} + {{\rm{O}}_2} + 2{{\rm{H}}_2}{\rm{O}} \to 2{{\rm{H}}_2}{\rm{S}}{{\rm{O}}_4} $ | (1) |
具体的反应过程为:
| $ {\rm{S}}{{\rm{O}}_2} + {\rm{C}} \to {\rm{S}}{{\rm{O}}_2} - {\rm{C}} $ | (2) |
| $ {{\rm{O}}_2} + {\rm{C}} \to {\rm{C}} - {\rm{O}} $ | (3) |
| $ {{\rm{H}}_2}{\rm{O}} + {\rm{C}} \to {\rm{C}} - {{\rm{H}}_2}{\rm{O}} $ | (4) |
| $ {\rm{C}} - {\rm{S}}{{\rm{O}}_2} + {\rm{C}} - {\rm{O}} \to {\rm{C}} - {\rm{S}}{{\rm{O}}_3} $ | (5) |
| $ {\rm{C}} - {\rm{S}}{{\rm{O}}_3} + {\rm{C}} - {{\rm{H}}_2}{\rm{O}} \to {\rm{C}} - {{\rm{H}}_2}{\rm{S}}{{\rm{O}}_4} $ | (6) |
| $ {\rm{C}} - {{\rm{H}}_2}{\rm{S}}{{\rm{O}}_4} + n{\rm{C}} - {{\rm{H}}_2}{\rm{O}} \to {\rm{C}} - \left( {{{\rm{H}}_2}{\rm{S}}{{\rm{O}}_4} \cdot n{{\rm{H}}_2}{\rm{O}}} \right) $ | (7) |
C为活性炭表面的活性位,-表示吸附作用。
这些硫酸沉积于活性炭中,然后蒸发回收硫酸,使活性位空出使得吸附过程循环进行。如日本三井矿业公司开发的Mitsui-BF工艺以活性炭脱除锅炉烟气、工业窑炉烟气以及化学工厂释放烟气中的SOx,并取得了巨大的成功[7]。
活性炭脱硝也是一个化学吸附和物理吸附同时存在的过程,当烟气中有SO2时,吸附过程中在活性位上的反应机理为[8]:
| $ {\rm{S}}{{\rm{O}}_2} + * \to {\rm{S}}{{\rm{O}}_2} * $ | (8) |
| $ 2{\rm{NO + }}{{\rm{O}}_2} + * \to 2{\rm{N}}{{\rm{O}}_2} * $ | (9) |
| $ {{\rm{O}}_2} + * \to {{\rm{O}}_2} * $ | (10) |
| $ {\rm{NO + }}{{\rm{O}}_2} * + {\rm{S}}{{\rm{O}}_2} * \to \left[ {\left( {{\rm{N}}{{\rm{O}}_2}} \right)\left( {{\rm{S}}{{\rm{O}}_3}} \right)} \right] * + * $ | (11) |
*表示活性位点上吸附的分子,总的反应方程式为:
| $ {\rm{S}}{{\rm{O}}_2} + 3{\rm{NO}} + 2{{\rm{O}}_2} + 2{{\rm{H}}_2}{\rm{O}} \to {{\rm{H}}_2}{\rm{S}}{{\rm{O}}_4} + 2{\rm{HN}}{{\rm{O}}_3} + {\rm{NO}} $ | (12) |
炭材料的表面物种如:酚羟基、羧基、羰基和内酯基等官能团,是其潜在的活性中心,对炭的脱硫性能影响显著。Karatepe等对活性炭吸附SO2的研究发现:吸附过程主要发生在活性炭的次微孔内,而活性炭表面的酚类和内酯类含氧官能团能够显著影响活性炭对SO2的吸附量[9]。对HNO3氧化的活性炭模拟烟气脱硫实验表明,氧化后的活性炭脱硫性能明显提高,活性炭表面C=O和C—O—官能团与活性炭脱硫性能关系密切[10]。经过HNO3改性后的活性焦脱硫脱硝效果提高显著[11]。Sousa以三聚氰胺作为氮源,向活性炭表面引入含氮官能团,发现修饰后活性炭催化活性显著提高,作者认为活性的提高与引入的含氮官能团具有孤对电子,更容易活化氧分子[12]。国内山西煤化所的程尚增等采用尿素修饰活性炭,制备了表面具有含氮活性物种的活性炭,发现吡啶氮有利于SO2的吸收[13]。北京大学的Yan等对活性半焦烟气脱硫的研究也发现,氧分子在活性半焦表面通过解离吸附产生的含氧中间产物是促进SO2氧化成SO3和H2SO4的关键[14]。但是,由于炭基材料表面物种受其制备方法、制备条件、原材料等因素的影响,其催化活性往往不高。
实际上,金属氧化物催化剂表面的含氮活性物种(如:氨基、酰胺基、吡啶、吡咯和季氮等官能团)也会影响催化剂的活性。大量研究表明含氮表面物种能够直接提高炭材料的催化活性[15],如含氮中孔活性炭具有更高活性和选择性,可以在低温条件下实现了H2S的氧化过程[16]。这主要是由于炭材料中的氮能够提高材料的电子传导能力和功函数[17]。而量子化学的研究结果证实,吡啶氮和季氮官能团能够提高材料活化分子氧的能力[18]。此外,当铁组分和氮同时掺杂到炭表面,铁可以和特定的含氮物种形成Fe-Nx结构。其中,铁和2个吡啶氮成键形成Fe—N2[19],铁和4个吡咯氮形成Fe—N4[20],如图 2所示。
在炭表面负载金属氧化物是提高脱硫活性的重要方法[2]。表 2给出了金属氧化物改性活性炭吸附SO2的相关研究的列表。对活性炭分别负载Fe、Co、Ni、V、Mn、Cu和Ce的脱硫活性评价表明,金属组分均能不同程度地提高活性炭的催化活性,其中负载V、Fe和Cu的催化剂表现出了优异的脱硫性能[21]。对Ni/活性炭催化剂的研究也发现,Ni能大幅度提高脱硫活性,并且随着Ni含量的增加,脱硫活性逐渐提高[22],对催化剂表面Ni的化学态分析表明,Ni/活性炭催化剂的活性中心主要是Ni和NiO[6],其中NiO的催化活性最高[23]。
| 碳源 | 吸附条件 | 金属氧化物 种类 |
浸渍方法 | φ(SO2)/ (×106) |
饱和 时间/min |
穿透 时间/min |
参考 文献 |
| 棕榈仁壳 | 气体入口流速0.15 L/min, 150 ℃, 1.013×105 Pa,1.0 g AC |
Ce | 湿法浸渍 Ce(NO3)3·6H2O |
1 000~2 500 | 455~245 | [24] | |
| 稻壳灰 | 气体入口流速0.15 L/min, 150 ℃, 1.013×105 Pa,0.7 g AC |
Ca+ Ce | 湿法浸渍 Ce(NO3)3·6H2O和干法CaO |
2 000 | 64 | 52 | [25] |
| 煤炭(河南) | 90 ℃,环境大气压,固定量的活性炭 | Ni | 湿法浸渍Ni(NO3)3·6H2O | 2 300 | 466.2 | [26] | |
| 商业活性炭 | 80 ℃,环境大气压,固定量的活性炭 | Mn | 湿法浸渍 Mn(NO3)2 |
2 800 | 185 | [27] | |
| 污泥 | 进气流速0.1 L/min, 环境压力,1.0 g AC | Ni | 湿法浸渍 Ni(NO3)2 |
1 200 | 71 | 30 | [28] |
| 胡桃壳 | 90 ℃,环境大气压,固定量的活性炭 | TiO2+Fe2O3,Ti | 干法浸渍 干法浸渍 |
2 0002 000 | 1 321.81 429.8 | [29] | |
| 商业活性炭 | 进气流速1.2 L/min, 80 ℃,1.013×105 Pa, 16.0 g AC | Fe(Ⅱ) | 湿法浸渍 Fe(NO3)2·9H2O |
2 700 | 545 | [30] | |
| 椰子壳 | 进气流速0.4 L/min, 50 ℃, 1.013×105 Pa | Cu | 湿法浸渍 Cu(NO3)2·3H2O |
2 000 | 42 | [31] | |
| 活性焦 | 120 ℃, 环境压力,1.0 g AC | Ce, B和Na | 湿法浸渍 Ce, Ba(NO3)2和Na2CO3 |
1 000 | 100 | 82 | [32] |
| 椰子壳 | 进气流速1.2 L/min, 150 ℃, 1.013×105 Pa, 0.01 g AC | V, Mn, Cu | 湿法浸渍 V(OH)5, Mn(OH)2和Cu(OH)2 |
200 | [33] | ||
| 烟煤 | 80 ℃,环境压力,16.0 g AC | Mn | 干法浸渍 | 2 000 | 35 | [34] | |
| 莱赛尔纤维 | 进气流速1.5 L/min, 环境温度和压力, 0.1 g AC | Cu | 湿法浸渍 Cu(NO3) 2·3H2O |
40 | 35.91 | 15.98 | [35] |
金属组分的掺杂提高了炭的脱硫性能,但其活性仍受表面含氧物种的影响。这主要体现在2方面:1)表面含氧物种能够改善金属组分在炭表面的分散情况;2)通过特定的表面含氧物种活化氧分子,促进SO2的氧化。Rau发现HNO3、H2SO4处理的活性炭引入的含氧官能团可以分散表面的金属活性组分,提高催化剂的脱硫效率[36]。郭家秀等的研究证实了上述结论[37],同时发现Mn/活性炭表面的C=O和C—O官能团可以转移O的孤对电子,活化吸附在催化剂表面的氧分子形成O2-,促进SO2氧化成SO3[38],而脱硫产生的硫酸盐也可以被炭还原,实现催化剂的再生[39]。此外,Fan采用共混合法制备的CO2O3、Ni2O3、CuO和V2O5催化剂用于脱除SO2的试验也发现,催化剂脱硫性能同时受金属组分和活性炭表面碱性官能团的影响[40]。对硝酸氧化后的活性炭负载Fe的脱硫活性研究发现,活性炭表面的C=O有助于提高催化活性,催化剂的活性中心是Fe3O4[41],Fe/活性炭的XPS谱图和XRD谱图证实,活性炭表面O=C—OH的含量以及Fe3O4的结晶度和催化剂脱硫活性关系密切[42]。Arcibar-Orozco对活性炭负载纳米Fe吸附SO2的机理研究提示,活性炭表面的醌类和色烯类官能团等带负电的碱性官能团与SO2的氧化过程密切相关[3]。由此可见,炭表面的碱性含氧物种有助于提高金属氧化物脱除SO2的性能。
4 活性炭负载金属氧化物对脱除氮氧化物的影响对于金属氧化物和NOx脱除的关系,也有金属氧化物可以增强活性中心的类似的观点[43]。表 3列出了迄今为止改性活性炭同时吸附SO2和NOx的研究。从表 2和表 3中的文献可知,通过在活性炭表面引入官能团或者催化剂来增加活性炭的活性位,提高了活性炭与SO2、NOx之间的作用力进而提高了吸附速率和催化转化活性。相对于比表面积,活性炭种类,活性炭的表面化学性质对其脱硫性能的影响更大。
| 炭源 | 气体组成 | 改性方法 | SO2穿透 时间/min |
NOx穿透 时间/min |
SO2饱和容量/ (mg·g-1) |
NOx饱和容量/ (mg·g-1) |
参考 文献 |
| 煤基炭 | 1 450×10-6 SO2, 400×10-6 NO, 450×10-6 NH3, φ(H2O)=2.5, φ(O2)=3.9 | NH4VO3 | 150 | 0 | 46 | [49] | |
| 商业活性炭 | 600×10-6 SO2, 650×10-6 NO, 200×10-6 CO, 11%CO2, 9.5%O2 | Na2CO3+KOH | 732 | 600 | 52 | 31 | [50] |
| 煤 | 1 500×10-6 SO2, 500×10-6 NO, 500×10-6 NH3, 2%H2O vapour, 5%O2 | V2O5 | 77 | 0 | [51] | ||
| 煤 | 1 500×10-6 SO2, 500×10-6 NO, 500×10-6 NH3, 3.5%H2O, 3.4%O2 | V2O5 | 200 | 10 | 119.8 | [52] | |
| 棕榈壳 | 1 000×10-6 SO2, 500×10-6 NO, 10%O2 | Ni(NO3)3·6H2O | 150 | 170 | [53] | ||
| 棕榈壳 | 1 000×10-6 SO2, 100×10-6 NO, 10%O2 | Ce(NO3)3·6H2O | 455 | 405 | 89.33 | 0.93 | [54] |
| 谷壳灰 | 2 000×10-6 SO2, 500×10-6 NO, 10%O2 | Ce(NO3)3·6H2O+CaO | 52 | 110 | 46.33 | [25] | |
| 污水污泥 | 2 000×10-6 SO2, 500×10-6 NO, 10%O2, 3.5%H2O | 壳聚糖 | 200 | 45 | 29.5 | 3.0 | [54] |
| 活性焦 | 1 000×10-6 SO2, 600×10-6 NOx, 15%O2, 3.5%H2O | Ce(NO3)3+Ba(NO3)2+Na2CO3 | 84 | 13 | [32] |
周亚端等研究发现金属氧化物组合改性活性焦的脱硝性能明显高于单一氧化物改性活性焦[44]。在150 ℃反应温度下,CuO和Fe2O3组合改性活性焦效果最好。陈立杰等通过对改性活性焦的制备以及吸附性能的研究发现,金属氧化物的加入在一定程度上可以提高活性焦的脱硫脱氮性能及再生性能[45]。常连成等采用浸渍法制备改性活性焦脱除烟气中的NOx,发现5%的FeSO4改性制得的活性焦效果最好[46]。李远涛等采用浸渍法改性活性炭,分析了单组分Cu和双组分Cu-Ni金属氧化物对活性炭改性后同时脱硫脱硝性能的影响。结果表面,单独CuO改性活性炭可以提高120 ℃下活性炭同时脱硫脱硝性能,且6%CuO/AC效果最好[47]。Pasel等对活性炭负载过渡金属氧化物(Fe、Cu和Cr)和催化剂上NH3的选择催化还原NO的性能作了研究,发现Fe2O3/AC在140~340 ℃的范围内脱硝率达100%[48]。
5 结语对电厂以及酸性气体的主要排放者来说,烟气脱硫和脱硝的必要性促使人们寻求更为有效、经济的技术。作为传统的脱硫脱硝的替代技术,采用经过表面改性的活性炭吸附脱硫脱硝方法已经越来越引起研究者的广泛关注。对于活性炭吸附脱硫脱硝,今后的研究方向可以重点的围绕以下方面。
1) 加强对改性活性炭脱硫脱硝机理研究,建立反应过程中的动力学模型,探讨金属氧化物改性活性炭吸附烟气中的SO2和NOx的控制机理。
2) 活性炭负载金属氧化物催化剂可以有效脱除烟气中的SO2与NOx,炭表面负载金属氧化物催化剂能够提高脱硫脱硝活性。但是炭表面的含氧物种、含氮物种对金属氧化物的脱硫活性与脱硝活性仍不明确。活性炭表面含氧活性物种、含氮活性物种对活性炭脱除SO2与NOx的性能影响可以在以后的研究中进一步探索和解决。
3) 活性炭的寿命一直是这一技术可持续应用的瓶颈,如何提高催化剂的耐热、机械和催化性能以及使用寿命也是以后的研究当中需要解决的问题。
| [1] |
Ye W, Li Y, Kong L, et al. Feasibility of flue-gas desulfurization by manganese oxides[J]. Transactions of Nonferrous Metals Society of China, 2013, 23(10): 3089-3094. DOI:10.1016/S1003-6326(13)62838-1 |
| [2] |
Abdulrasheed A A, Jalil A A, Triwahyono S, et al. Surface modification of activated carbon for adsorption of SO2 and NOx: A review of existing and emerging technologies[J]. Renewable and Sustainable Energy Reviews, 2018, 94: 1067-1085. DOI:10.1016/j.rser.2018.07.011 |
| [3] |
Arcibar-Orozco J A, Rangel-Mendez J R, Bandosz T J. Reactive adsorption of SO2 on activated carbons with deposited iron nanoparticles[J]. Journal of Hazardous Materials, 2013, 246/247: 300-309. DOI:10.1016/j.jhazmat.2012.12.001 |
| [4] |
马双忱, 赵毅, 马宵颖, 等. 活性炭床加微波辐射脱硫脱硝的研究[J]. 热能动力工程, 2006, 21(4): 338-341, 433. Ma Shuangchen, Zhao Yi, Ma Xiaoying, et al. A study of the desulfuration and denitration on active carbon beds provided with microwave irradiation[J]. Journal of Engineering for Thermal Energy and Power, 2006, 21(4): 338-341, 433. DOI:10.3969/j.issn.1001-2060.2006.04.002 (in Chinese) |
| [5] |
石清爱, 于才渊. 改性活性炭的烟气脱硫脱硝性能研究[J]. 化学工程, 2010, 38(10): 106-109. Shi Qing'ai, Yu Caiyuan. Study on modified activated carbon for flue gas desulphurization and denitrification[J]. Chemical Engineering(China), 2010, 38(10): 106-109. DOI:10.3969/j.issn.1005-9954.2010.10.022 (in Chinese) |
| [6] |
Mochida I, Korai Y, Shirahama M, et al. Removal of SOx and NOx over activated carbon fibers[J]. Carbon, 2000, 38(2): 227-239. DOI:10.1016/S0008-6223(99)00179-7 |
| [7] |
Olson D G, Tsuji K, Shiraishi I. The reduction of gas phase air toxics from combustion and incineration sources using the MET-Mitsui-BF activated coke process[J]. Fuel Processing Technology, 2000, 65/66: 393-405. DOI:10.1016/S0378-3820(99)00106-X |
| [8] |
Silas K, Ghani W A W A K, Choong T S Y, et al. Breakthrough studies of Co3O4 supported activated carbon monolith for simultaneous SO2/NO removal from flue gas[J]. Fuel Processing Technology, 2018, 180: 155-165. DOI:10.1016/j.fuproc.2018.08.018 |
| [9] |
Karatepe N, Orbak I·, Yavuz R, et al. Sulfur dioxide adsorption by activated carbons having different textural and chemical properties[J]. Fuel, 2008, 87(15/16): 3207-3215. |
| [10] |
Guo J, Liang J, Chu Y, et al. Desulfurization activity of nickel supported on acid-treated activated carbons[J]. Applied Catalysis A:General, 2012, 421/422: 142-147. DOI:10.1016/j.apcata.2012.02.010 |
| [11] |
雷晶晶, 强敏, 杨娟娟, 等. 改性柱状活性焦用于烟气脱硫脱硝的研究[J]. 工业安全与环保, 2014, 40(7): 92-95. Lei Jingjing, Qiang Min, Yang Juanjuan, et al. Study on desulfurization and denitrification by modified columnar activated-coke[J]. Industrial Safety and Environmental Protection, 2014, 40(7): 92-95. DOI:10.3969/j.issn.1001-425X.2014.07.029 (in Chinese) |
| [12] |
Sousa J P S, Pereira M F R, Figueiredo J L. Modified activated carbon as catalyst for NO oxidation[J]. Fuel Processing Technology, 2013, 106: 727-733. DOI:10.1016/j.fuproc.2012.10.008 |
| [13] |
程尚增, 郭倩倩, 黄张根, 等. 纤维素制备渗氮炭材料用于脱除烟气中的SO2[J]. 高等学校化学学报, 2015, 36(6): 1126-1132. Cheng Shangzeng, Guo Qianqian, Huang Zhanggen, et al. Cellulose generated carbon materials with nitrogen doping for the desulfurization of flue gas[J]. Chemical Journal of Chinese Universities, 2015, 36(6): 1126-1132. (in Chinese) |
| [14] |
Yan Z, Liu L, Zhang Y, et al. Activated semi-coke in SO2 removal from flue gas:Selection of activation methodology and desulfurization mechanism study[J]. Energy & Fuels, 2013, 27(6): 3080-3089. |
| [15] |
Shen W, Fan W. Nitrogen-Containing porous carbons:Synthesis and application[J]. J Mater Chem A, 2013, 1(4): 999-1013. DOI:10.1039/C2TA00028H |
| [16] |
Sun F, Liu J, Chen H, et al. Nitrogen-Rich mesoporous carbons:highly efficient, regenerable metal-free catalysts for low-temperature oxidation of H2S[J]. ACS Catalysis, 2013, 3(5): 862-870. DOI:10.1021/cs300791j |
| [17] |
Wiggins-Camacho J D, Stevenson K J. Effect of nitrogen concentration on capacitance, density of states, electronic conductivity, and morphology of N-doped carbon nanotube electrodes[J]. The Journal of Physical Chemistry C, 2009, 113(44): 19082-19090. DOI:10.1021/jp907160v |
| [18] |
Saidi W A. Oxygen reduction electrocatalysis using N-doped graphene quantum-dots[J]. The Journal of Physical Chemistry Letters, 2013, 4(23): 4160-4165. DOI:10.1021/jz402090d |
| [19] |
Lefèvre M, Dodelet J P, Bertrand P. O2 reduction in PEM fuel cells:Activity and active site structural information for catalysts obtained by the pyrolysis at high temperature of Fe precursors[J]. The Journal of Physical Chemistry B, 2000, 104(47): 11238-11247. DOI:10.1021/jp002444n |
| [20] |
Bouwkamp-Wijnoltz A L, Visscher W, van Veen J A R, et al. On active-site heterogeneity in pyrolyzed carbon-supported iron porphyrin catalysts for the electrochemical reduction of oxygen:An in situ Mössbauer study[J]. The Journal of Physical Chemistry B, 2002, 106(50): 12993-13001. DOI:10.1021/jp0266087 |
| [21] |
Gao X, Liu S, Zhang Y, et al. Physicochemical properties of metal-doped activated carbons and relationship with their performance in the removal of SO2 and NO[J]. Journal of Hazardous Materials, 2011, 188(1/2/3): 58-66. |
| [22] |
Chu Y, Guo J, Liang J, et al. Ni supported on activated carbon as catalyst for flue gas desulfurization[J]. Science China Chemistry, 2010, 53(4): 846-850. DOI:10.1007/s11426-010-0087-y |
| [23] |
Guo J, Liang J, Chu Y, et al. Influence of Ni species of Ni/AC catalyst on its desulfurization performance at low temperature[J]. Chinese Journal of Catalysis (Chinese Version), 2010, 31(3): 278-282. DOI:10.3724/SP.J.1088.2010.90908 |
| [24] |
Sumathi S, Bhatia S, Lee K T, et al. Adsorption isotherm models and properties of SO2 and NO removal by palm shell activated carbon supported with cerium (Ce/PSAC)[J]. Chemical Engineering Journal, 2010, 162(1): 194-200. DOI:10.1016/j.cej.2010.05.028 |
| [25] |
Dahlan I, Lee K T, Kamaruddin A H, et al. Sorption of SO2 and NO from simulated flue gas over rice husk ash (RHA)/CaO/CeO2 sorbent:Evaluation of deactivation kinetic parameters[J]. Journal of Hazardous Materials, 2011, 185(2/3): 1609-1613. |
| [26] |
Guo J, Liang J, Chu Y, et al. Desulfurization activity of nickel supported on acid-treated activated carbons[J]. Applied Catalysis A:General, 2012, 421/422: 142-147. DOI:10.1016/j.apcata.2012.02.010 |
| [27] |
Qu Y, Guo J, Chu Y, et al. The influence of Mn species on the SO2 removal of Mn-based activated carbon catalysts[J]. Applied Surface Science, 2013, 282: 425-431. DOI:10.1016/j.apsusc.2013.05.146 |
| [28] |
Wu C, Song M, Jin B, et al. Adsorption of sulfur dioxide using nickel oxide/carbon adsorbents produced by one-step pyrolysis method[J]. Journal of Analytical and Applied Pyrolysis, 2013, 99: 137-142. DOI:10.1016/j.jaap.2012.10.011 |
| [29] |
Guo J, Fan L, Peng J, et al. Desulfurization activity of metal oxides blended into walnut shell based activated carbons[J]. Journal of Chemical Technology & Biotechnology, 2014, 89(10): 1565-1575. |
| [30] |
Liu X, Guo J, Chu Y, et al. Desulfurization performance of iron supported on activated carbon[J]. Fuel, 2014, 123: 93-100. DOI:10.1016/j.fuel.2014.01.068 |
| [31] |
Yi H, Zuo Y, Liu H, et al. Simultaneous removal of SO2, NO, and CO2 on metal-modified coconut shell activated carbon[J]. Water, Air, & Soil Pollution, 2014. DOI:10.1007/s11270-014-1965-2 |
| [32] |
Zuo Y, Yi H, Tang X. Metal-Modified active coke for simultaneous removal of SO2 and NOx from sintering flue gas[J]. Energy & Fuels, 2015, 29(1): 377-383. |
| [33] |
Chiu C H, Lin H, Kuo T, et al. Simultaneous control of elemental mercury/sulfur dioxide/nitrogen monoxide from coal-fired flue gases with metal oxide-impregnated activated carbon[J]. Aerosol and Air Quality Research, 2015, 15(5): 2094-2103. DOI:10.4209/aaqr.2015.03.0176 |
| [34] |
Yang L, Jiang X, Yang Z, et al. Effect of MnSO4 on the removal of SO2 by manganese-modified activated coke[J]. Industrial & Engineering Chemistry Research, 2015, 54(5): 1689-1696. |
| [35] |
Bai B, Lee C W, Lee Y S, et al. Metal impregnate on activated carbon fiber for SO2 gas removal:Assessment of pore structure, Cu supporter, breakthrough, and bed utilization[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2016, 509: 73-79. |
| [36] |
Rau J Y, Tseng H H, Chiang B C, et al. Evaluation of SO2 oxidation and fly ash filtration by an activated carbon fluidized-bed reactor:The effects of acid modification, copper addition and operating condition[J]. Fuel, 2010, 89(3): 732-742. |
| [37] |
Qu Y, Guo J, Chu Y, et al. The influence of Mn species on the SO2 removal of Mn-based activated carbon catalysts[J]. Applied Surface Science, 2013, 282: 425-431. DOI:10.1016/j.apsusc.2013.05.146 |
| [38] |
Guo J, Qu Y, Shu S, et al. Effects of preparation conditions on Mn-based activated carbon catalysts for desulfurization[J]. New Journal of Chemistry, 2015, 39(8): 5997-6015. DOI:10.1039/C5NJ00873E |
| [39] |
Liu Y, Qu Y, Guo J, et al. Thermal regeneration of manganese supported on activated carbons treated by HNO3 for desulfurization[J]. Energy & Fuels, 2015, 29(3): 1931-1940. |
| [40] |
Fan L, Jiang X, Jiang W, et al. Physicochemical properties and desulfurization activities of metal oxide/biomass-based activated carbons prepared by blending method[J]. Adsorption, 2014, 20(5/6): 747-756. |
| [41] |
Liu X, Guo J, Chu Y, et al. Desulfurization performance of iron supported on activated carbon[J]. Fuel, 2014, 123: 93-100. DOI:10.1016/j.fuel.2014.01.068 |
| [42] |
Guo J, Liu X, Luo D, et al. Influence of calcination temperatures on the desulfurization performance of Fe supported activated carbons treated by HNO3[J]. Industrial & Engineering Chemistry Research, 2015, 54(4): 1261-1270. |
| [43] |
Kante K, Deliyanni E, Bandosz T J. Interactions of NO2 with activated carbons modified with cerium, lanthanum and sodium chlorides[J]. Journal of Hazardous Materials, 2009, 165(1/2/3): 704-713. |
| [44] |
周亚端, 向晓东, 熊友沛, 等. 金属氧化物组合改性活性焦脱硝性能的研究[J]. 环境工程, 2013, 31(3): 90-92, 121. Zhou Yaduan, Xiang Xiaodong, Xiong Youpei, et al. Study on denitrification performance of modified activated coke by metal-oxides[J]. Environmental Engineering, 2013, 31(3): 90-92, 121. (in Chinese) |
| [45] |
陈立杰, 王恩德, 苏永渤. 改性活性焦的制备及吸附性能研究[J]. 安全与环境学报, 2004, 4(4): 73-75. Chen Lijie, Wang Ende, Su Yongbo. On how to prepare a kind of activated modified coke and its absorption capacity[J]. Journal of Safety and Environment, 2004, 4(4): 73-75. DOI:10.3969/j.issn.1009-6094.2004.04.022 (in Chinese) |
| [46] |
常连成, 肖军, 张辉, 等. 改性活性焦低温脱硝实验研究[J]. 太原理工大学学报, 2010, 41(5): 593-597. Chang Liancheng, Xiao Jun, Zhang Hui, et al. Experimental study on denitrification by modified activated coke at low temperature[J]. Journal of Taiyuan University of Technology, 2010, 41(5): 593-597. (in Chinese) |
| [47] |
李远涛, 易红宏, 唐晓龙, 等. Ni-Cu金属氧化物改性活性炭同时脱硫脱硝性能研究[J]. 工业安全与环保, 2017, 43(5): 4-8. Li Yuantao, Yi Honghong, Tang Xiaolong, et al. Study on simultaneous desulfurization and denitrification of Ni-Cu metal oxide modified activated carbon[J]. Industrial Safety and Environmental Protection, 2017, 43(5): 4-8. DOI:10.3969/j.issn.1001-425X.2017.05.002 (in Chinese) |
| [48] |
Pasel J, Käβner P, Montanari B, et al. Transition metal oxides supported on active carbons as low temperature catalysts for the selective catalytic reduction (SCR) of NO with NH3[J]. Applied Catalysis B:Environmental, 1998, 18(3/4): 199-213. |
| [49] |
Wang Y, Liu Z, Zhan L, et al. Performance of an activated carbon honeycomb supported V2O5 catalyst in simultaneous SO2 and NO removal[J]. Chemical Engineering Science, 2004, 59(22/23): 5283-5290. |
| [50] |
Zhu J, Wang Y, Zhang J, et al. Experimental investigation of adsorption of NO and SO2 on modified activated carbon sorbent from flue gases[J]. Energy Conversion and Management, 2005, 46(13/14): 2173-2184. |
| [51] |
郭彦霞, 刘振宇, 李允梅, 等. 氨再生条件对V2O5/AC同时脱硫脱硝活性的影响[J]. 燃料化学学报, 2007, 35(3): 344-348. Guo Yanxia, Liu Zhenyu, Li Yunmei, et al. NH3 regeneration of SO2-captured V2O5/AC catalyst-sorbent for simultaneous SO2 and NO removal[J]. Journal of Fuel Chemistry and Technology, 2007, 35(3): 344-348. DOI:10.3969/j.issn.0253-2409.2007.03.018 (in Chinese) |
| [52] |
Ma J, Liu Z, Liu Q, et al. SO2 and NO removal from flue gas over V2O5/AC at lower temperatures:Role of V2O5 on SO2 removal[J]. Fuel Processing Technology, 2008, 89(3): 242-248. DOI:10.1016/j.fuproc.2007.11.003 |
| [53] |
Sumathi S, Bhatia S, Lee K T, et al. Performance of an activated carbon made from waste palm shell in simultaneous adsorption of SOx and NOx of flue gas at low temperature[J]. Science in China Series E:Technological Sciences, 2009, 52(1): 198-203. DOI:10.1007/s11431-009-0031-6 |
| [54] |
Fan X, Zhang X. Simultaneous removal of SO2 and NO with activated carbon from sewage sludge modified by chitosan[J]. Applied Mechanics and Materials, 2012, 253/254/255: 960-964. |
 2020, Vol. 37
2020, Vol. 37