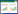英国BP公司2019年世界能源统计年鉴表明,煤炭仍是中国能源消费中的主要原料,2018年中国煤炭消费重现0.9%的增长,占全球煤炭消费总量的50.5%[1]。由于火力发电是我国最主要的发电形式,锅炉是最主要的供能设备,短期煤炭在我国能源结构中的主导地位不会发生根本改变。然而,煤炭燃烧产生的烟气中含有NOx、SO2及烟尘,表 1为近年废气中的主要污染物排放情况。NOx与SO2是造成酸雨、雾霾、光化学烟雾的主要原因,同时也对人体呼吸系统产生危害[2-6]。2018年中国生态环境状况公报显示酸雨区面积占国土面积的5.5%[7]。
| Year | SO2 | NOx | Dust |
| 2013 | 2 043.92 | 2 227.34 | 1 278.14 |
| 2014 | 1 974.42 | 2 078.00 | 1 740.75 |
| 2015 | 1 859.12 | 1 851.02 | 1 538.01 |
| 2016 | 1 102.86 | 1 394.31 | 1 010.66 |
| 2017 | 875.40 | 1 258.83 | 796.26 |
2015年中国实施新的《环境保护法》,并发布了《全面实施燃煤电厂超低排放和节能改造工作方案》,要求提前完成超低排放改造任务。我国已经进入环境保护攻坚战阶段,污染物排放限值及对排污收费的增加,急需采取多污染物同时控制技术来应对烟气中SO2和NOx的排放。新兴的多种污染物联合协同脱除技术可在低温下实现污染物的同时脱除[9],且具有效率高、占地面积小、对烟道改造小等优点,近年来已成为烟气综合治理领域的研究热点[10]。
目前燃煤锅炉烟气后污染控制技术脱硫与脱硝独立进行。常见的脱硝工艺包括选择性非催化还原(SNCR)[11]、选择性催化还原(SCR)[12]和微波脱硝[13]等,其中SCR用氨将NOx还原为N2,脱硝效率达90%,是最有效的NOx控制技术之一。脱硫工艺分为湿法脱硫(WFGD)[14]、干法脱硫(DFGD)[15-16]及半干法脱硫(SDFGD)[17]。WFGD是基于钙基吸收的脱硫工艺,脱硫效率95%以上。单独脱除技术成熟,对每一种污染物有较高的脱除效率,工业上常采用SNCR、SCR与WFGD串联,分级脱除NOx与SO2,导致烟气处理装置占地面积大,系统繁杂,投资及运维费用较高[18]。近年,单一或混合紧凑的同时脱硫脱硝技术成为研究热点,本论文对烟气同时脱硝脱硫方法和研究状况进行评述。
1 脱硝脱硫方法 1.1 液相氧化吸收法液相氧化吸收法是选用适当的氧化剂,必要时辅以其他添加剂配制成复合溶液,以实现对烟气中NOx和SO2的协同吸收。该技术需要考虑气态污染物在溶液中的溶解度、气液接触时间和气液比。传统的湿法洗涤工艺中常使用CO(NH2)2及NaOH溶液作为吸收液,其对吸收SO2非常有效,但是因NO低的水溶性导致其脱硝效率很差。
为有效去除烟气中的NOx,需在溶液中加入氧化剂,已报道的氧化剂包括NaClO2[3]、NaClO[19]、ClO2[20]、Fenton试剂[21]、Na2S2O8[22]、Ca(ClO)2[23]、腐殖酸钠(HA-Na)[23]、H2O2[24]或它们的混合物。烟气中NOx主要是NO和NO2,其中NO含量超过90%。在气液相界面处,氧化剂会将NO氧化成高价态的NOx(NO2、NO3、HNO3及N2O5)。这些高价态的N物种具有良好的水溶性,易于被水吸收转变成NO2-和NO3-,SO2被转化成SO42-。在吸收过程中,气液界面大小和吸收剂组成对提高烟气的脱除效率有至关重要的作用,目前对液相氧化技术的研究主要集中在新的复合吸收剂或新型的湿法技术以增加气液界面。
NaClO2是廉价的氧化剂,其良好的水溶解性及较高的氧化效率而得到广泛的应用。Lee等[25]用NaClO2溶液在湿壁塔中进行了同时脱硫脱硝,在气液接触时间5 s,液气比7.7 L/m3时,NOx和SO2脱除率分别为67%和100%。在NaClO2溶液中添加尿素、HA-Na、NaBr、NaClO和H2O2等辅助添加剂均提高NOx脱除率。Fang等[26]用NaClO2(质量分数为1%)溶液和尿素(质量分数为5%)在逆流接触的填料塔中NOx脱除率为93%。Hao等[27]由NaClO2(质量分数为0.7%)和Ha-Na(质量分数为4%)组成的复合吸收剂,NOx脱除率达98%。
Park等[3]采用安装有超声波加湿器的湿式静电除尘器(如图 1),包括2个工艺步骤:1)超声波加湿器将NaClO2溶液雾化吸收烟气中的NOx及SO2;2)静电沉积除雾。超声波加湿器产生的液滴比雾化喷嘴产生的液滴小,相比传统的湿法洗涤,超声波加湿器可使气液界面最大化,可缩短气液接触时间(1 s)和降低液气比(0.1 L/m3),提高脱除效率,减少运行费用。Pourmohammadbagher等[28]在大型涡流系统中通过内部轴向板和高速电机(转速1 800 r/min)减小液滴尺寸(气体流速:300 Nm3/h);NaClO2溶液喷洒到内部风扇上,转板将喷洒的溶液剪切成较小的液滴;内部风扇的湍流也有助于形成小液滴。这种湿洗设计可实现高效率的工业应用。
高级氧化法是利用光、声、电等方式原位产生·OH等强氧化性粒子来降解有机物,该技术在废水处理领域研究广泛[29]。近年,利用紫外高级氧化工艺(UV-AOPs)同时脱硫脱硝的研究也不断增多[30],UV-AOP方法主要包括UV/H2O2[31]、UV/过硫酸盐(PS)[32]、UV/过硫酸氢钾[33]等;其主要是用羟基自由基(HO·)和硫酸盐自由基(SO4-·)来除去NO和SO2。常见的反应器形式是在充满H2O2溶液的鼓泡反应器安装紫外线灯,通过紫外线照射H2O2产生·OH,烟气与生成的·OH发生氧化反应并被吸收,如图 2所示。
| $ {{\rm{H}}_2}{{\rm{O}}_2}\mathop \to \limits^{hv} 2 \cdot {\rm{OH}} $ | (1) |
Liu等[29]在鼓泡反应器中使用波长为254 nm的紫外线灯辐射H2O2溶液,NO和SO2的脱除率分别为73%和100%。Liu等[34]在UV-AOPs方法中采用在H2O2溶液中添加10 mmol/L的H2O2/NaOH复合溶液,表明NO的脱除率显著提高。Hao等[35]将汽化的H2O2溶液与气态污染物被同时引入紫外辐照反应器中,在最适宜条件下,SO2去除率为100%,NO去除率为87.8%,此工艺仍需深入研究。
在254 nm时,H2O2和PS的量子产率只有0.5和0.7 mol·Einstein-1,相对较低;因此,深度去除NO所需的氧化剂剂量较高,限制了其工业应用。次氯酸盐(ClO-)量子产率(约1.0 mol·Einstein-1),在降解污染物和微污染物方面具有优异的性能[36-37]。UV/ClO-产生的主要自由基是HO·和Cl·如方程(2)所示。
| $ {\rm{HOCI}}\mathop \to \limits^{hv} {\rm{HO}} \cdot + {\rm{CI}} $ | (2) |
Yang[38]和Liu等[39]分别用UV/NaClO和UV/Ca(ClO)2同时脱除SO2和NO,NO的脱除率分别为95.6%和92.4%,同时由于溶液的碱度高,SO2去除率达100%。其认为SO2的存在促进形成HClO,进一步提高HO·的产量。因此,ClO-是光催化脱除SO2和NO的合适的自由基前体。Hao等[28]用紫外/亚氯酸钠(UV/NaClO2)同时脱除SO2和NO,NH4OH作为添加剂被用来抑制ClO2和NO2的生成,SO2和NO去除率分别为98.7%和99.1%。
1.3 低温等离子体法(NTP)低温等离子体技术(NTP)是具有前途的大气污染物控制方法。该技术主要采用介质阻挡放电(DBD)、电晕放电(CD)和脉冲电晕放电(PCD)等方式,将气体污染物转化为惰性物质或可处理物质的一种成功的方法。等离子体区域的高能电子可直接撞击O2分子或H2O分子产生O·、·OH和HO2·等自由基,这些自由基可将NOx和SO2氧化成硝酸和硫酸,涉及的自由基氧化反应在很短的时间内即可终止(小于10-3 s)[40]。因此,NTP工艺与传统工艺相比,在同时脱硫脱硝的系统紧凑性上具有一定优势。
由于介电材料或者脉冲波形更能增加电子能量,因此与电晕放电相比,DBD和PCD的使用更为普遍。一般情况下,H2O和空气常用来放电产生O·和·OH。在单独NTP体系中,NOx和SO2的脱除不彻底,高能耗是获得较高脱除效率的必要条件。Yu等[41]采用PCD处理烟气,在停留时间为4 s,能量密度为65 J/L时,SO2脱除率为91.8%,NOx脱除率仅有55.8%。研究表明,NTP与湿法洗涤过程或催化剂相结合可提高污染物脱除效率,且能耗明显降低[42-43]。Wang等[42]将电晕反应器处理后的气体通入硫酸盐与硫代硫酸盐混合溶液中,SO2被全部去除,NOx脱除率达到71%。Pham等[43]在DBD反应器中装填TiO2(如图 3),在放电电压12 kV,放电频率为600 Hz,停留时间1 s时,SO2和NO脱除率分别为100%和85%。为降低能耗,基于NTP技术的混合系统可成为工业应用示范的先进工艺。
电子束烟气处理技术(EBFGT)是在同时脱硫脱硝领域不断发展的工业应用技术。EBFGT工艺通常包括水冷却系统、电子加速器、电子束辐照室和静电除尘器或袋式除尘器。含有水蒸气和空气的烟气被电子束照射产生·OH、HO2·和O(3P)等氧化性基团,NOx和SO2通过式(3)~(7)等自由基氧化反应被氧化成HNO3和H2SO4[44-45]。NH3或NH4OH也可以与烟气一起注入电子辐照室,与生成的硝酸和硫酸反应形成固相副产物硝酸铵和硫酸铵,利用静电除尘器或袋式除尘器收集后,作为肥料。
| $ {\rm{NO + O}}\left( {^3{\rm{P}}} \right) + {\rm{M}} \to {\rm{N}}{{\rm{O}}_2} + {\rm{M}} $ | (3) |
| $ {\rm{NO + H}}{{\rm{O}}_2} \cdot + {\rm{M}} \to {\rm{N}}{{\rm{O}}_2} + \cdot {\rm{OH}} + {\rm{M}} $ | (4) |
| $ {\rm{N}}{{\rm{O}}_2} + \cdot {\rm{OH}} + {\rm{M}} \to {\rm{HN}}{{\rm{O}}_3} + {\rm{M}} $ | (5) |
| $ {\rm{S}}{{\rm{O}}_2} + \cdot {\rm{OH}} + {\rm{M}} \to \cdot {\rm{HS}}{{\rm{O}}_3} + {\rm{M}} $ | (6) |
| $ \cdot {\rm{HS}}{{\rm{O}}_3} + {{\rm{O}}_2} \to {\rm{S}}{{\rm{O}}_3} + {\rm{H}}{{\rm{O}}_2} \cdot $ | (7) |
| $ {\rm{S}}{{\rm{O}}_3} + {{\rm{H}}_2}{\rm{O}} \to {{\rm{H}}_2}{\rm{S}}{{\rm{O}}_4} $ | (8) |
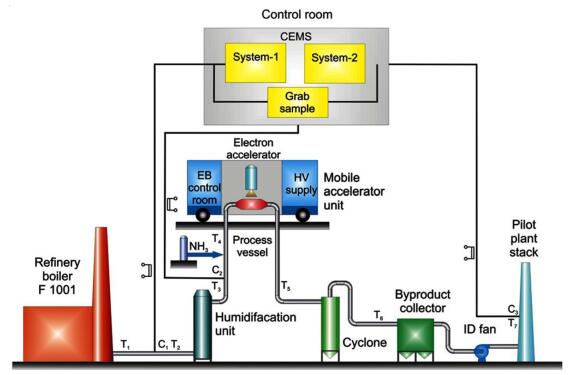
日本原子能研究所(JAERI)与Ebara公司联合研究了EBFGT工艺(如图 4所示),通过中试验证了工程方案的新思路,包括双纵向气体辐照和NH3注入系统等。结果证明,NOx和SO2脱除率分别为80%和99%,电除尘器中得到的副产物(NH4)2SO4质量分数为76%~92%,NH4NO3质量分数为0.8%~ 15.0%,可用来生产复合肥[46]。2011年,土耳其实施了VGSⓇEBFGT工艺,其独特之处在于在没有静电除尘器或袋式过滤器的情况下,实现烟气的同时处理和固体副产物的回收[47]。VGSⓇEBFGT由3部分组成,上部是烟气喷射和电子束辐照室,中间是由NH3和水雾进行吸收的化学反应器,最下面是气液分离器。在适宜条件下,NOx和SO2的脱除率分别为81%和99%,产物中(NH4)2SO4与NH4NO3干基质量分数为45%~73%和9%~31%。
与其他工艺相比,EBFGT技术是具有竞争力的商业化烟气同步净化工艺,不仅可高效处理烟气,副产品为高质量的肥料,且没有二次污染。然而,该技术较高的能耗及运行费用限制了其在普通锅炉烟气处理工艺上的应用。
1.5 臭氧前置氧化+化学吸收法对比上述方法可知,烟气中SO2的脱除相对容易,难点在于其中NOx的脱除。占NOx含量95%以上的NO在水和碱中的溶解度非常低,但其它高价态的NOx具有好的水溶性,且氮的价态越高,NOx的溶解度越大,因此,N2O5在水中具有最高的溶解度[48]。鉴于此,将低价态的NO氧化为高价态的NOx然后进行有效脱除的方法应运而生。
O3是强氧化剂,其还原产物为O2,不产生二次污染。近期,研究学者对O3前置氧化结合不同吸收剂进行同时脱硫脱氮展开了大量研究,均取得了较佳的脱除效果。该工艺是对烟道内的烟气喷射臭氧,将其中的NO氧化为高价态的NOx,然后与WFGD相结合同时脱硫脱硝。喷射的O3量不同,NO的氧化产物不同。Sun等[49]采用原位红外技术对O3氧化NO产物进行分析,发现当n(O3)/n(NO)≤1时,氧化产物主要为NO2;n(O3)/n(NO)>1时,部分NO2被进一步氧化为NO3和N2O5等。其他研究人员的实验与模拟均与此结果一致[50-54]。
表 2列举具有代表性的O3氧化结合化学吸收的结果,该工艺主要集中在吸收系统所用的吸收剂及吸收反应器的优化,旨在促进气液传质过程,提高脱硫脱硝效果及工业应用价值。Sun等[6]在鼓泡反应器中采用软锰矿浆吸收O3氧化后的NOx和SO2,得到较高烟气净化效果的同时将软锰矿中的Mn提取出来,优质的副产品MnSO4·H2O具有较高的商业价值。孙承朗[56]采用氧化镁作为吸收剂,吸收副产物MgSO3再生后可以实现循环利用,但其脱硝效率没有竞争力。孙宝昌等[57]采用旋转固定床来强化传质过程,并且在NaOH溶液中添加NaClO、H2O2和KMnO4后,脱硝效率明显提高,但添加KMnO4、NaClO会导致吸收液中盐的复杂化,导致后续利用困难。郭少鹏[54]采用氨水在鼓泡反应器中吸收,氨基吸收剂的优点在于廉价易得,且副产物可以作为优质的肥料,具有较高的综合利用价值。王春波等[58]采用喷淋散射技术(如图 5所示)结合臭氧前置氧化进行同时脱硫脱硝实验,探究了O3与NO物质的量比、SO2初始体积分数、液气体积比、浸没深度等对脱硫和脱硝效率的影响。适宜条件下脱硫率为99.2%,脱硝率接近80%。姚淑美[59]分别采用鼓泡和喷淋散射技术研究了臭氧前置氧化以及SO2浓度、水蒸气含量、CO2、吸收液温度及氨水中的添加剂等因素的影响,SO2去除率大于99%,NOx去除率大于89%。臭氧氧化脱硝的反应机理已经基本明确,影响氧化吸收效率的因素也逐渐明晰,各方面均已取得较大突破。结合该技术的工艺优势,其工业化进程必将加速,实现大规模工业应用指日可待。
| Reactor type | Absorbents | MR(O3/NO) | Removal efficiencies/% | Ref. | |
| NOx | SO2 | ||||
| Bubbling reactor | Pyrolusite slurry | 1.2 | 82 | 90 | [6] |
| Bubbling reactor | NaOH | 1.0 | 90 | 100 | [53] |
| Bubbling reactor | NH4OH | 1.0 | 90 | 99 | [54] |
| Wet scrubbing | Ca(OH)2 | 1.6 | 97 | 100 | [9] |
| Wet atomizing | H2O2 | 1.8 | 89 | 100 | [55] |
| Wet scrubbing | MgO slurry | 1.0 | 76 | 98 | [56] |
| spray and bubbling scattered column | Ca(OH)2 | 1.0 | 80 | 99 | [58] |
| spray and bubbling scattered column | NH4OH | 1.0 | >89 | >99 | [59] |
| 注:MR(O3/NO)为O3与NO的物质的量之比。 | |||||

|
| 图 5 喷淋散射技术示意图 Fig.5 Schematic diagram of the spray and bubbling scattered column |
| |
干法催化再生技术是在克服选择性催化还原及湿法脱硫基础上发展起来的一种干法同时脱除技术。该技术一般采用移动床反应器,主要分为烟气催化脱除及催化剂再生2个步骤:在烟气催化脱除阶段,NO在氨的作用下被选择性催化还原为N2,同时SO2被吸附在催化剂表面;当催化剂中SO2达到饱和后,催化剂转入再生器中,并与氨反应生成(NH4)2SO3固体,由此实现NO和SO2的同时脱除,并使催化剂再生循环利用。
中科院山西煤化所研究的CuO/Al2O3基催化剂体系及活性炭基催化剂体系取得了较高的脱硫脱硝效率(>85%)及硫资源转化率(>85%)。近年来,研究者对其进行了改进研究,Ma等[60]采用微波辐照活性炭基催化剂,在微波功率为420 W的条件下,锌系催化剂和锰系催化剂的催化效果较为明显,去除率可达95%左右。此外,光催化在同时脱硫脱硝领域的研究也取得了不错的效果。Xia等[61]采用BiOI/Al2O3催化剂,在滴流反应器中实现了NO和SO2同时光催化去除,脱除效率接近100%,该反应器在长期重复间歇运行中可以保持较高的脱硫脱硝效率,光催化性能较为稳定。
2 结语与展望我国煤炭消费量依然很大,随着烟气排放标准日趋严格,企业的生存与发展正面临巨大的挑战。现有的烟气处理技术各有各的优势,但也存在许多不足之处(表 3)。新兴的臭氧前置氧化脱硝结合化学吸收同时处理烟气技术,脱硫脱硝效率较高,结合氨基吸收剂进行吸收可获得硫酸铵和硝酸铵或石膏等副产物,资源利用率较高,具有相当大的发展潜力;且该技术对企业已有设备的改动较小,只需增设臭氧发生器和O3喷射口,便可实现新工艺在旧设备中的运行。臭氧前置氧化的氨水吸收技术已在山西潞安进行了侧线试验,工业化示范装置也在安装中,将为燃煤污染脱除一体化提供样板。
| 烟气处理技术 | 优势 | 存在的问题 |
| 液相氧化吸收法 | 设备简单,易操作,脱除效率较高 | 氧化剂吸收剂消耗较大,易造成二次污染,设备易腐蚀 |
| 紫外高级氧化法 | 脱除效率较高,过程易于控制 | 设备运行成本较高,工业放大较困难 |
| 低温等离子体法 | 脱除效率较高,设备紧凑,无废水废渣等二次污染 | 能耗相对较高,设备维护工作量大 |
| 电子束法 | 设备简单,操作方便,过程易控制,脱除效率较高,副产物价值高 | 设备能耗高,维护工作量大 |
| 臭氧前置氧化+化学吸收法 | 脱除效率较高,无二次污染,副产物价值高 | 制备臭氧成本较高 |
| 干法催化再生技术 | 脱除效率较高,催化剂可循环利用 | 设备复杂,催化剂易中毒,催化剂研发周期长,工业化进程缓慢 |
| [1] |
BP集团. BP世界能源统计年鉴[R].英国: BP集团, 2019
|
| [2] |
Chang G, Song C, Wang L. A modeling and experimental study of flue gas desulfurization in a dense phase tower[J]. Journal of Hazardous Materials, 2011, 189(1/2): 134-140. |
| [3] |
Park H W, Choi S, Park D W. Simultaneous treatment of NO and SO2 with aqueous NaClO2 solution in a wet scrubber combined with a plasma electrostatic precipitator[J]. Journal of Hazardous Materials, 2015, 285: 117-126. DOI:10.1016/j.jhazmat.2014.11.040 |
| [4] |
Ye J, Shang J, Li Q, et al. The use of vacuum ultraviolet irradiation to oxidize SO2 and NOx for simultaneous desulfurization and denitrification[J]. Journal of Hazardous Materials, 2014, 271: 89-97. DOI:10.1016/j.jhazmat.2014.02.011 |
| [5] |
Duarte J H, Fanka L S, Costa J A V. Utilization of simulated flue gas containing CO2, SO2, NO and ash for Chlorella fusca cultivation[J]. Bioresource Technology, 2016, 214: 159-165. DOI:10.1016/j.biortech.2016.04.078 |
| [6] |
Sun W, Ding S, Zeng S, et al. Simultaneous absorption of NOx and SO2 from flue gas with pyrolusite slurry combined with gas-phase oxidation of NO using ozone[J]. Journal of Hazardous Materials, 2011, 192(1): 124-130. |
| [7] |
中华人民共和国环境保护部.中国环境状况公报2018[R].中国: 中华人民共和国环境保护部, 2019
|
| [8] |
中华人民共和国国家统计局. 中国统计摘要-2014[M]. 北京: 中国统计出版社, 2014.
|
| [9] |
Wang Z, Zhou J, Zhu Y, et al. Simultaneous removal of NOx, SO2 and Hg in nitrogen flow in a narrow reactor by ozone injection:Experimental results[J]. Fuel Processing Technology, 2007, 88(8): 817-823. DOI:10.1016/j.fuproc.2007.04.001 |
| [10] |
Skalska K, Miller J S, Wilk M, et al. Nitrogen oxides ozonation as a method for NOx emission abatement[J]. Ozone:Science & Engineering, 2012, 34(4): 252-258. |
| [11] |
Hu Z, Jiang E, Ma X. Numerical simulation on operating parameters of SNCR process in a municipal solid waste incinerator[J]. Fuel, 2019, 245: 160-173. DOI:10.1016/j.fuel.2019.02.071 |
| [12] |
Ye B, Lee M, Jeong B, et al. Partially reduced graphene oxide as a support of Mn-Ce/TiO2 catalyst for selective catalytic reduction of NOx with NH3[J]. Catalysis Today, 2019, 328: 300-306. DOI:10.1016/j.cattod.2018.12.007 |
| [13] |
Segawa T, Kawaguchi K, Ishii K, et al. Nickel oxide powder synthesis from aqueous solution of nickel nitrate hexahydrate by a microwave denitration method[J]. Advanced Powder Technology, 2015, 26(3): 983-990. DOI:10.1016/j.apt.2015.04.004 |
| [14] |
Bian J, Zhang Q, Min X, et al. Modified clinoptilolite catalysts for seawater flue gas desulfurization application:Preparation, characterization and kinetic evaluation[J]. Process Safety and Environmental Protection, 2016, 101: 117-123. DOI:10.1016/j.psep.2015.10.018 |
| [15] |
Chen Z, Dong H, Yu H, et al. In-situ electrochemical flue gas desulfurization via carbon black-based gas diffusion electrodes:Performance, kinetics and mechanism[J]. Chemical Engineering Journal, 2017, 307: 553-561. DOI:10.1016/j.cej.2016.08.116 |
| [16] |
Ding S, Li Y, Zhu T, et al. Regeneration performance and carbon consumption of semi-coke and activated coke for SO2 and NO removal[J]. Journal of Environmental Sciences, 2015, 34: 37-43. DOI:10.1016/j.jes.2015.02.004 |
| [17] |
Li Y, Yi H, Tang X, et al. Study on the performance of simultaneous desulfurization and denitrification of Fe3O4-TiO2 composites[J]. Chemical Engineering Journal, 2016, 304: 89-97. DOI:10.1016/j.cej.2016.06.035 |
| [18] |
Wang L, Zhao W, Wu Z. Simultaneous absorption of NO and SO2 by FeII EDTA combined with Na2SO3 solution[J]. Chemical Engineering Journal, 2007, 132(1/2/3): 227-232. |
| [19] |
Mondal M K, Chelluboyana V R. New experimental results of combined SO2 and NO removal from simulated gas stream by NaClO as low-cost absorbent[J]. Chemical Engineering Journal, 2013, 217: 48-53. DOI:10.1016/j.cej.2012.12.002 |
| [20] |
Jin D, Deshwal B R, Park Y S, et al. Simultaneous removal of SO2 and NO by wet scrubbing using aqueous chlorine dioxide solution[J]. Journal of Hazardous Materials, 2006, 135(1/2/3): 412-417. |
| [21] |
Zhao Y, Han Y, Guo T, et al. Simultaneous removal of SO2, NO and Hg0 from flue gas by ferrate (Ⅵ) solution[J]. Energy, 2014, 67: 652-658. DOI:10.1016/j.energy.2014.01.081 |
| [22] |
Khan N E, Adewuyi Y G. Absorption and oxidation of nitric oxide (NO) by aqueous solutions of sodium persulfate in a bubble column reactor[J]. Industrial & Engineering Chemistry Research, 2010, 49(18): 8749-8760. |
| [23] |
Raghunath C V, Pandey P, Saini R, et al. Absorption of SO2 and NO through an integrative process with a cost-effective aqueous oxidant[J]. Perspectives in Science, 2016, 8: 699-701. DOI:10.1016/j.pisc.2016.06.063 |
| [24] |
Liémans I, Thomas D. Simultaneous NOx and SOx reduction from oxyfuel exhaust gases using acidic solutions containing hydrogen peroxide[J]. Energy Procedia, 2013, 37: 1348-1356. DOI:10.1016/j.egypro.2013.06.010 |
| [25] |
Lee H K, Deshwal B R, Yoo K S. Simultaneous removal of SO2 and NO by sodium chlorite solution in wetted-wall column[J]. Korean Journal of Chemical Engineering, 2005, 22(2): 208-213. DOI:10.1007/BF02701486 |
| [26] |
Fang P, Cen C, Tang Z, et al. Simultaneous removal of SO2 and NOx by wet scrubbing using urea solution[J]. Chemical Engineering Journal, 2011, 168(1): 52-59. DOI:10.1016/j.cej.2010.12.030 |
| [27] |
Hao R, Zhang Y, Wang Z, et al. An advanced wet method for simultaneous removal of SO2 and NO from coal-fired flue gas by utilizing a complex absorbent[J]. Chemical Engineering Journal, 2017, 307: 562-571. DOI:10.1016/j.cej.2016.08.103 |
| [28] |
Pourmohammadbagher A, Jamshidi E, Ale-Ebrahim H, et al. Study on simultaneous removal of NOx and SO2with NaClO2in a novel swirl wet system[J]. Industrial & Engineering Chemistry Research, 2011, 50(13): 8278-8284. |
| [29] |
Liu Y, Zhang J, Sheng C, et al. Simultaneous removal of NO and SO2 from coal-fired flue gas by UV/H2O2 advanced oxidation process[J]. Chemical Engineering Journal, 2010, 162(3): 1006-1011. DOI:10.1016/j.cej.2010.07.009 |
| [30] |
Hao R, Mao X, Qian Z, et al. Simultaneous removal of SO2 and NO using a novel method of ultraviolet irradiating chlorite-ammonia complex[J]. Environmental Science & Technology, 2019, 53(15): 9014-9023. |
| [31] |
Liu Y, Zhang J. Photochemical oxidation removal of NO and SO2 from Simulated flue gas of coal-fired power plants by wet scrubbing using UV/H2O2 advanced oxidation process[J]. Industrial & Engineering Chemistry Research, 2011, 50(7): 3836-3841. |
| [32] |
Liu Y, Wang Y, Wang Q, et al. Simultaneous removal of NO and SO2 using vacuum ultraviolet light (VUV)/heat/peroxymonosulfate (PMS)[J]. Chemosphere, 2018, 190: 431-441. DOI:10.1016/j.chemosphere.2017.10.020 |
| [33] |
Liu Y, Xu W, Zhao L, et al. Absorption of NO and simultaneous absorption of SO2/NO using a vacuum ultraviolet light/ultrasound/KHSO5 system[J]. Energy & Fuels, 2017, 31(11): 12364-12375. |
| [34] |
Liu Y, Wang Q, Yin Y, et al. Advanced oxidation removal of NO and SO2 from flue gas by using ultraviolet/H2O2/NaOH process[J]. Chemical Engineering Research and Design, 2014, 92(10): 1907-1914. DOI:10.1016/j.cherd.2013.12.015 |
| [35] |
Hao R, Zhao Y, Yuan B, et al. Establishment of a novel advanced oxidation process for economical and effective removal of SO2 and NO[J]. Journal of Hazardous Materials, 2016, 318: 224-232. DOI:10.1016/j.jhazmat.2016.06.052 |
| [36] |
Guo K, Wu Z, Shang C, et al. Radical chemistry and structural relationships of PPCP degradation by UV/chlorine treatment in simulated drinking water[J]. Environmental Science & Technology, 2017, 51(18): 10431-10439. |
| [37] |
Sun P, Lee W N, Zhang R, et al. Degradation of DEET and caffeine under UV/chlorine and simulated sunlight/chlorine conditions[J]. Environmental Science & Technology, 2016, 50(24): 13265-13273. |
| [38] |
Yang S, Pan X, Han Z, et al. Kinetics of nitric oxide absorption from simulated flue gas by a wet UV/chlorine advanced oxidation process[J]. Energy & Fuels, 2017, 31(7): 7263-7271. |
| [39] |
Liu Y, Wang Y, Liu Z, et al. Oxidation removal of nitric oxide from flue gas using UV photolysis of aqueous hypochlorite[J]. Environmental Science & Technology, 2017, 51(20): 11950-11959. |
| [40] |
Sun Y, Zwolińska E, Chmielewski A G. Abatement technologies for high concentrations of NOx and SO2 removal from exhaust gases:A review[J]. Critical Reviews in Environmental Science and Technology, 2016, 46(2): 119-142. DOI:10.1080/10643389.2015.1063334 |
| [41] |
Yu C, Xu F, Luo Z, et al. Influences of water vapor and fly ash addition on NO and SO2 gas conversion efficiencies enhanced by pulsed corona discharge[J]. Journal of Electrostatics, 2009, 67(6): 829-834. DOI:10.1016/j.elstat.2009.06.003 |
| [42] |
Wang M, Sun Y, Zhu T. Removal of NOx, SO2, and Hg from simulated flue gas by plasma-absorption hybrid system[J]. IEEE Transactions on Plasma Science, 2013, 41(2): 312-318. DOI:10.1109/TPS.2012.2234483 |
| [43] |
Pham H C, Kim K S. Effect of TiO2 thin film thickness on NO and SO2 removals by dielectric barrier discharge-photocatalyst hybrid process[J]. Industrial & Engineering Chemistry Research, 2013, 52(15): 5296-5301. |
| [44] |
Kikuchi R, Pelovski Y. Low-dose irradiation by electron beam for the treatment of high-SOx flue gas on a semi-pilot scale:Consideration of by-product quality and approach to clean technology[J]. Process Safety and Environmental Protection, 2009, 87(2): 135-143. DOI:10.1016/j.psep.2008.10.003 |
| [45] |
Calinescu I, Martin D, Chmielewski A, et al. E-Beam SO2 and NOx removal from flue gases in the presence of fine water droplets[J]. Radiation Physics and Chemistry, 2013, 85: 130-138. DOI:10.1016/j.radphyschem.2012.10.008 |
| [46] |
Pawelec A, Chmielewski A G, Licki J, et al. Pilot plant for electron beam treatment of flue gases from heavy fuel oil fired boiler[J]. Fuel Processing Technology, 2016, 145: 123-129. DOI:10.1016/j.fuproc.2016.02.002 |
| [47] |
Tan E, Vnal S, Doğan A, et al. New "wet type" electron beam flue gas treatment pilot plant[J]. Radiation Physics and Chemistry, 2016, 119: 109-115. DOI:10.1016/j.radphyschem.2015.10.007 |
| [48] |
Van Durme J, Dewulf J, Leys C, et al. Combining non-thermal plasma with heterogeneous catalysis in waste gas treatment:A review[J]. Applied Catalysis B:Environmental, 2008, 78(3/4): 324-333. |
| [49] |
Sun C, Zhao N, Zhuang Z, et al. Mechanisms and reaction pathways for simultaneous oxidation of NOx and SO2 by ozone determined by in situ IR measurements[J]. Journal of Hazardous Materials, 2014, 274: 376-383. DOI:10.1016/j.jhazmat.2014.04.027 |
| [50] |
Lin F, Wang Z, Ma Q, et al. N2O5 formation mechanism during the ozone-based low-temperature oxidation deNOx process[J]. Energy & Fuels, 2016, 30(6): 5101-5107. |
| [51] |
Wang H, Zhuang Z, Sun C, et al. Numerical evaluation of the effectiveness of NO2 and N2O5 generation during the NO ozonation process[J]. Journal of Environmental Sciences, 2016, 41: 51-58. DOI:10.1016/j.jes.2015.05.015 |
| [52] |
Li B, Zhao J, Lu J. Numerical study of the simultaneous oxidation of NO and SO2 by ozone[J]. International Journal of Environmental Research and Public Health, 2015, 12(2): 1595-1611. DOI:10.3390/ijerph120201595 |
| [53] |
Zhang J, Zhang R, Chen X, et al. Simultaneous removal of NO and SO2 from flue gas by ozone oxidation and NaOH absorption[J]. Industrial & Engineering Chemistry Research, 2014, 53(15): 6450-6456. |
| [54] |
Guo S, Lv L, Zhang J, et al. Simultaneous removal of SO2 and NOx with ammonia combined with gas-phase oxidation of NO using ozone[J]. Chemical Industry and Chemical Engineering Quarterly, 2015, 21(2): 305-310. DOI:10.2298/CICEQ140618029G |
| [55] |
Yoon H J, Park H W, Park D W. Simultaneous oxidation and absorption of NOx and SO2 in an integrated O3 oxidation/wet atomizing system[J]. Energy & Fuels, 2016, 30(4): 3289-3297. |
| [56] |
Sun C, Zhao N, Wang H, et al. Simultaneous absorption of NOx and SO2 using magnesia slurry combined with ozone oxidation[J]. Energy & Fuels, 2015, 29(5): 3276-3283. |
| [57] |
Sun B, Sheng M, Gao W, et al. Absorption of nitrogen oxides into sodium hydroxide solution in a rotating packed bed with preoxidation by ozone[J]. Energy & Fuels, 2017, 31(10): 11019-11025. |
| [58] |
严雪南, 王春波, 司桐, 等. 基于臭氧前置氧化的新型喷淋散射技术同时脱硫脱硝实验研究[J]. 动力工程学报, 2019, 39(6): 461-467, 485. Yan Xuenan, Wang Chunbo, Si Tong, et al. Experimental research on simultaneous removal of SO2 and NOx by a spray-and-bubbling scattered reactor based on ozone pre-oxidation[J]. Journal of Chinese Society of Power Engineering, 2019, 39(6): 461-467, 485. (in Chinese) |
| [59] |
姚淑美.等离子体制备臭氧与同时脱硫脱硝研究[D].天津: 天津大学, 2019 Yao Shumei. Study on preparation of ozone by plasma and simultaneous desulfurization and denitrification[D]. Tianjin: Tianjin University, 2019(in Chinese) |
| [60] |
Ma S, Liu W, Jin X, et al. Experimental study on simultaneous desulfurization and denitrification over activated carbon carried catalyst under microwave irradiation[J]. Advanced Materials Research, 2012, 347: 3540-3544. |
| [61] |
Xia D, Hu L, He C, et al. Simultaneous photocatalytic elimination of gaseous NO and SO2 in a BiOI/Al2O3-padded trickling scrubber under visible light[J]. Chemical Engineering Journal, 2015, 279: 929-938. DOI:10.1016/j.cej.2015.05.097 |
 2020, Vol. 37
2020, Vol. 37